Key Structures
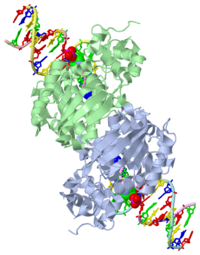
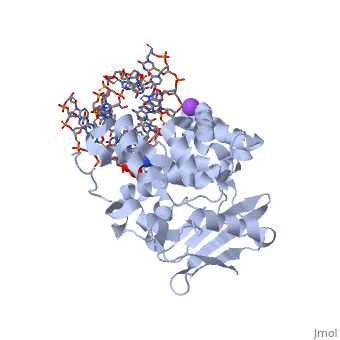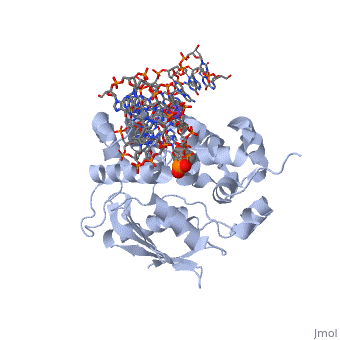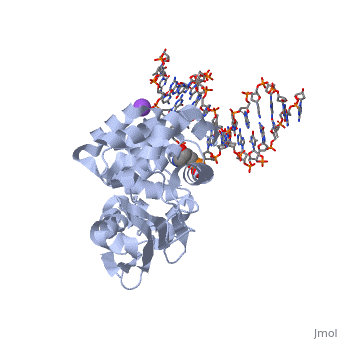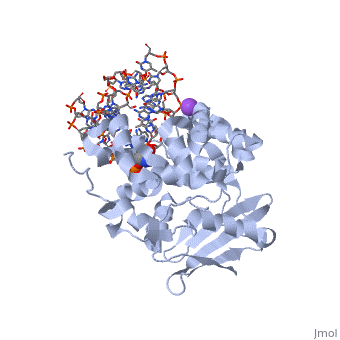
AlkA is composed of three main domains with dimensions of approximately 50 Angstroms, 45 Angstroms, and 25 Angstroms.[3] The first domain (residues 1-112) is the that is composed of a five stranded antiparallel beta sheet and two alpha helices.[3] The (residues 113-230) contains seven alpha helices that create a hydrophobic core.[3] The third domain (resudues 231-282) is the that contains a bundle of three alpha helices. [3] The three domains of AlkA create a distinct binding structure that is lined with electron rich aromatic residues to form a hydrophobic cleft. The cleft is found between domains 2 and 3, and forms the binding pocket for the alkylated base. The hydrophobic cleft has the ability to widen between domains 2 and 3 for accepting a variety of alkylated bases.[3]
AlkA is a member of the helix-hairpin-helix (HhH) family of DNA glycosylases, where two compact alpha helical structures are connected by a hairpin loop.[4] In AlkA, the is composed of residues 202-227 and is responsible for binding the damaged DNA by van der Waals interactions, a few hydrogen bonds, and metal ion interactions.
Active Sites and Mechanism
The HhH segment of AlkA connects to the phosphodiester backbone of DNA by hydrogen bonding and . The binding of the HhH segment to DNA stabilizes the damaged base and creates a 66 degree bend away from the protein that widens the minor groove of DNA.[4] fits into the minor groove between base pairs and allows the alkylated base to be flipped into the active site.[2]
The AlkA active site is located where the second and third domains are separated by a deep nonpolar cleft that is lined with the aromatic side chains Phe18, Tyr273, Trp272, Tyr222, and Trp218.[3] These side chains create a for the alkylated base.[3] Once the damaged base is in the active site, stabilizes the flipped out alkylated base in the binding pocket by aromatic ring-stacking interactions.[4] is essential for allowing the reaction to proceed, and points into the nonpolar pocket in order to allow stabilization of a carbocation intermediate. This stabilization is what allows the cleavage of the glycosidic bond on the damaged base.[4] Mutation or inhibition of the Asp238 residue will prevent AlkA from performing base excision repair.
DNA Interaction
The AlkA protein stabilizes DNA by polar and nonpolar interactions with the DNA backbone.
Molecular Level
Atomic Level
In order to keep the DNA strand bound to the protein, AlkA depends on van der Waals interactions on the minor groove of DNA. The van der Waals interactions play a major role in AlkA’s preference for double stranded DNA.[2] An example of these interactions may be seen with the Pro175 wedged into the minor groove of DNA and anchoring it by van der Waals interctions.[2]