Sandbox Reserved 761
From Proteopedia
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
|
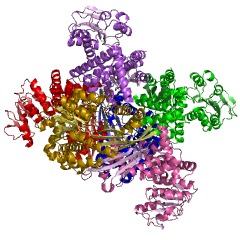
Glutamate Dehydrogenase (GDH) is an enzyme required for urea synthesis, that converts glutamate to α-ketoglutarate, and vice versa. Located in the mitochondria, GDH is an important branch-point enzyme between carbon and nitrogen metabolism. GDH catalyzes the reversible NAD (P)+-linked oxidative deamination of L-glutamate into alpha ketoglutarate and ammonia in two steps. The first step involves a Schiff base intermediate being formed between ammonia and alpha ketoglutarate. This Schiff base intermediate is crucial because it establishes the alpha carbon atom in glutamate’s stereochemistry. The second step involves the Schiff base intermediate being protonated, which is done by the transfer of a hydride ion from NADPH resulting in L-glutamate. GDH is unique because it is able to utilize both NAD+ and NADP+ (15). NADP+ is utilized in the forward reaction of alpha ketogluterate and free ammonia, which are converted to L-glutamate via a hydride transfer from NADPH to glutamate (15). NAD+ is utilized in the reverse reaction, which involves L-glutamate being converted to alpha ketoglutarate and free ammonia via an oxidative deamination reaction (1). The extensive production of ammonia by peripheral tissue or glutamate dehydrogenase is not allowed because of the highly toxic effects of circulating ammonia in cells. Therefroe, the ammonia produced in the reverse reaction of GDH is excreted as NH4+ in the urine, by first going through the urea cycle.