1lpa
From Proteopedia
| |||||||
| , resolution 3.04Å | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||
| Activity: | Triacylglycerol lipase, with EC number 3.1.1.3 | ||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
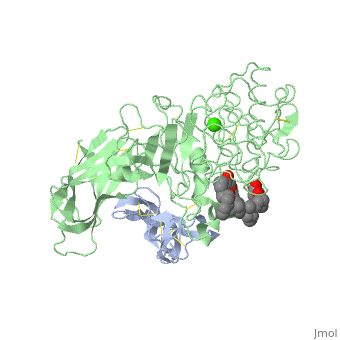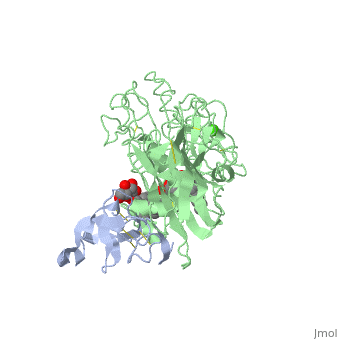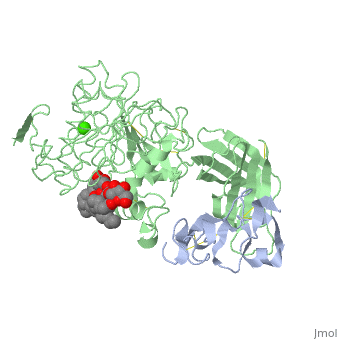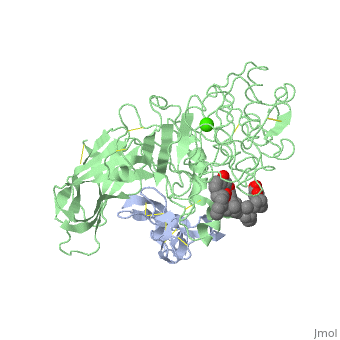
INTERFACIAL ACTIVATION OF THE LIPASE-PROCOLIPASE COMPLEX BY MIXED MICELLES REVEALED BY X-RAY CRYSTALLOGRAPHY
Overview
The three-dimensional structure of the lipase-procolipase complex, co-crystallized with mixed micelles of phosphatidylcholine and bile salt, has been determined at 3 A resolution by X-ray crystallography. The lid, a surface helix covering the catalytic triad of lipase, adopts a totally different conformation which allows phospholipid to bind to the enzyme's active site. The open lid is an essential component of the active site and interacts with procolipase. Together they form the lipid-water interface binding site. This reorganization of the lid structure provokes a second drastic conformational change in an active site loop, which in its turn creates the oxyanion hole (induced fit).
About this Structure
1LPA is a Protein complex structure of sequences from Homo sapiens and Sus scrofa. Full crystallographic information is available from OCA.
Reference
Interfacial activation of the lipase-procolipase complex by mixed micelles revealed by X-ray crystallography., van Tilbeurgh H, Egloff MP, Martinez C, Rugani N, Verger R, Cambillau C, Nature. 1993 Apr 29;362(6423):814-20. PMID:8479519
Page seeded by OCA on Sun Mar 30 22:05:37 2008