TmAFP is an hyperactive antifreeze protein and its origin is the Tenebrio Mollitor beetle.
The protein consists of 84 amino acids and the molecular weight is 8.4 kDA.
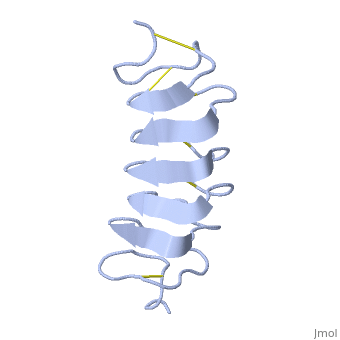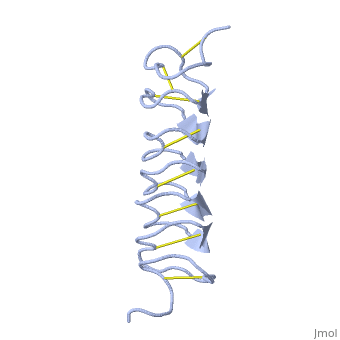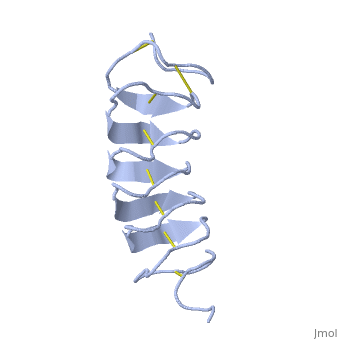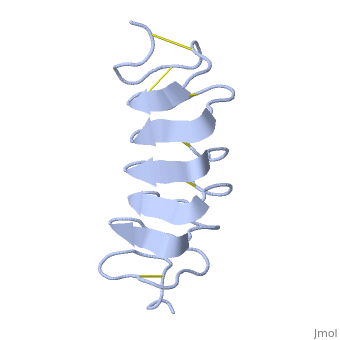
TmAFP composed of 7 tandem repeats which consist of 12 amino acids-(TCTxSxxCxxAx).
TCT or ACT motifs are aligned to form a flat along one side of the molecule the Beta sheets right handed which are the binding site of the protein.
The rest of the tandem repeat forms the loop which enables very organized structure of the protein.
Cysteine all over the tandem repeats, provide the which contribute to the stability of the protein.
six of the eight disulphide bounds construct near perfect alignment enables appropriate structure that allows binding to the ice lattice. The other do not fit this pattern.
The extraordinary tightness of the 12 amino-acids turn is also facilitated by intra-loop hydrogen bond connections between backbone CO and NH groups.
TmAFP is the smallest Beta-Helix with only 12 amino acids per turn. Therefore, it has a very narrow bore, which is constricted and further bisected by disulphide bonds to form two channels, leaving no room for hydrophobic core.
The few Hydrophobic residues have their side chains projecting outwards to the solvent. In the core there is room only for the relatively small side chains of the conserves Serine and Alanine to project into the core, on either side of the bisecting disulphide bridge.
[1] or to the article describing Jmol [2] to the rescue.
Function
Disease
Relevance
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.