Introduction
Interleukin 33 is a cytokine member of the interleukin 1 superfamily. Cytokines are proteins involved in cell signaling. Their role is to regulate the function and the activity of other cells. IL-33 is involved in innate and adaptive immune responses.
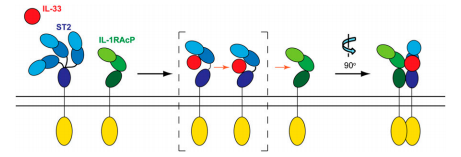
In 2005, IL-33 was identified as a ligand for the transmembrane receptor ST2 (also known as IL1RL1 and it IL-1 receptor accessory protein (IL-1RAcP) as you see below, both members of the interleukin-1 receptor family. IL-33/ST2 signaling is involved in T-cell immune responses.
We will focuse on the interaction bewteen IL33 and the ST2 ectodomain (as you can see ).
Function
Caracteristics of IL-33
IL-33 is constitutively expressed in several cells, such as epithelial or endothelial cells. IL-33 is also expressed in an inducible way by immune cells. In this case, the constitutive expression of IL-33 is very low or absent. In mast cells, the expression of IL-33 is induced by the calcium, but the mechanisms are still unclear. IL-33 is secreted in damaged tissues, where it activates the immune response.
Target cells
ST2 exists in a soluble form (sST2) and a transmembrane form (ST2L). The both forms can interact with IL-33. ST2L are expressed in T lymphocytes, when these lymphocytes are specializing into Th2 cells (T helper type 2 cells, a specific type of T lymphocytes). This specialization occurs in the presence of IL4, expected to be secreted by polynuclear basophils.
IL-33 interacts with the ectodomain of ST2L on Th2 cells. This interaction provokes the recruitment of the myeloid differentiation primary-response protein 88 (MYD88) complex, which is involved in the immune response against commune viral infections and some bacterial infections.
When IL-33 interacts with the sST2, it inhibits the fixation of IL-33 with the ectodomain of ST2.
Structure
Overall structure
The understanding of the interaction of IL-33 with its receptors has been discovered thanks to the determination of the crystal structure of IL-33 in complex with ectodomain of ST2.
Besides, the combination of crystallography and small-angle X-ray-scattering methods reveal that ST2 has a very flexible conformation, contrary to IL-1RAcP. In fact, ST2 is constituted of three IgG-like domains (). D1 and D2 gather to form a single D1D2 module, connected through a linker with the D3 domain. ST2 contains also linked with NAG and an which is involved in the interaction with the other receptor, IL-1RAcP.
This conformational specificity provides a capactity of ligand-binding with IL-33. Moreover, the rigidity of IL-1RAcP explains that it can not bind the IL-33 ligand directly.
In humans, IL-33 in its full length is composed of 270 residues and is biologically active.
The model includes IL-33 residues Ser117 to Ser268 and ST2 residues Ser21 to Arg317.
The structure of IL-33 consists in a forming a β-trefoil structure. It also contains an . Specific loops, such as the β4-β5 loop, are involved in the interaction with the accessory receptor IL-1RAcP when IL-33 is bound to ST2.
IL-33/ST2 interactions
In the complex, IL-33 interacts with the three domains of ST2. The binding interface is very large and composed of two separate sites.
In the , thirteen IL-33 residues from β-loops are in contact with the D1D2 module of ST2: the four marked acids residues of IL-33 form a salt-bridge with five marked ST2 residues respectively. Besides, form hydrogen bonds with main-chain atoms of ST2. IL-33 mutation of one of the acid residues (144, 148, 149 and 244) highly decreases the affinity of ST2 for IL-33.
In the , eight IL-33 residues from β-strands interact with the D3 domain of ST2.
There are both hydrophobic and hydrophilic interactions. Residues of IL-33 form an hydrophobic cluster (IL-33 residues Tyr163 and Leu182 and ST2 residues Leu246, Leu306, and Leu311). A salt-bridge interaction occurs between acidic residue respectivevly of IL-33 and ST2.

