This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Ferritin
From Proteopedia
|
Contents |
Function
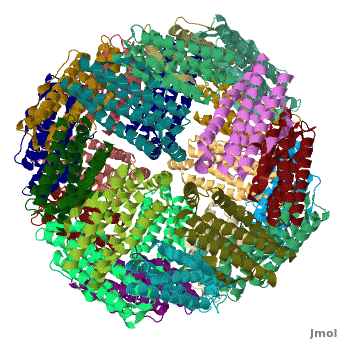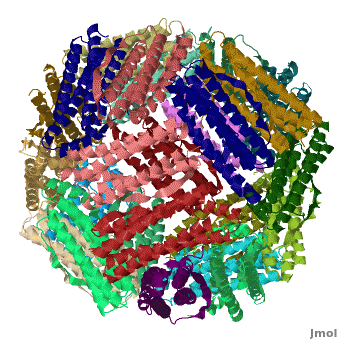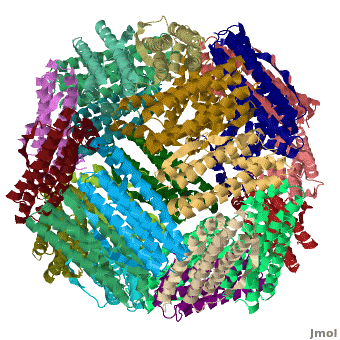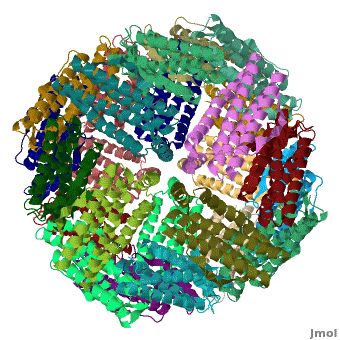
Ferritin (FR) is an iron storage and release protein. It stores iron as microcrystals with phosphate and hydroxide ions. FR is composed of 24 subunits of heavy chain (FTH) a[1]
nd light chain (FTL). Amphibians have an additional middle subunit FR (FTM).
- Bacterioferritin (BFR) structure is very similar to FR. It contains a binuclear iron center and haem. It stores iron as ferric oxide mineral in its hollow central cavity.
- Thioferritin (TFR) is a ferritin-related protein with 2 cysteine residues adjacent to the dimetal binding site.
- Another iron storing protein is the DNA-binding Protein of Starved cells (Dps) - a ferritin-like diiron carboxylate.
- MrgA – another iron storage protein - belongs to the Dps family.
Relevance
Cavities formed by FR are used for the manufacture of nanoparticles. FR is used as a marker for iron overload disorder.
Disease
FR deficiency can lead to anemia.
3D Structures of Ferritin
Updated on 21-January-2016
References
- ↑ Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: Past, present and future. Biochim Biophys Acta. 2010 Aug;1800(8):760-9. doi: 10.1016/j.bbagen.2010.03.011. , Epub 2010 Mar 19. PMID:20304033 doi:http://dx.doi.org/10.1016/j.bbagen.2010.03.011
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, David Canner, Eran Hodis, Alexander Berchansky