Isocitrate Lyase (ICL) is a metabolic enzyme that converts the metabolite isocitrate into glyoxylate and succinate. ICL is a homotetramer with each monomer being composed of 14 alpha helices, 14 beta sheets, and a magnesium ion cofactor. ICL has shown clinical relevance in the disease state Tuberculosis where it is responsible for the persistence of Mycobacterium tuberculosis during the chronic stage of infection. This survival strategy mediated by ICL is characterized by a metabolic shortcut within the Citric Acid Cycle. ICL creates this shunt pathway by converting isocitrate to succinate and glyoxylate, diverting acetyl-CoA from the beta-oxidation of fatty acids.
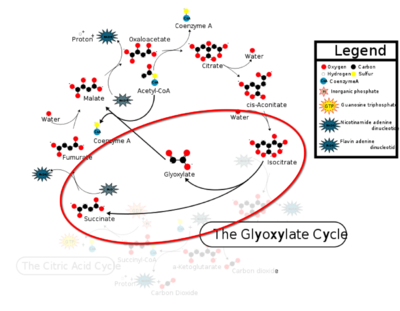
Figure 1: ICL mediated glyoxylate shunt pathway of the Citric Acid Cycle
Structure
The ICL homotetramer possesses 222 symmetry, with an axis of rotation at x-axis, y-axis, and z-axis of the enzyme. Two individual subunits off ICL are held together by a characteristic between three alpha helices formed by residues 370-384, 349-367, and 399-409 on neighboring monomers. The interlocking mechanism created by these helices provides additional strength to hold the two monomeric subunits together, allowing ICL to essentially be composed of two dimerized subunits. This interaction will bury approximately 18% of the surface of each subunit, and will help to shield the interior binding site from hydration.
Active Site
Mechanism
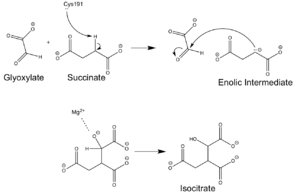
Chemical Mechanism of Isocitrate Lyase