Function
Disease
Relevance
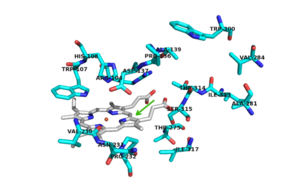
Structural highlights
blue - n terminus hook
magenta - n terminus
light pink - c terminus
There are 6 conserved key active site residues that suround the . These residues are Arg 104, Trp 107, His 108, His 270, Asp 381.
The location of the binding site for isoniazid (INH) is located near the delta meso heme edge, about 3.8 A away from the heme iron. This binding site is found within what is considered to be the usual substrate access channel of peroxidases. The reaction between INH and the enzyme must occur from interaction in a binding site intended for the natural substrate. Asp 137 plays a key role in the activation and binding of INH. Asp 137 creates energetically favorable interactions due to its ability to make hydrogen-bond interactions between its carboxylic acid side chain and the pyridinyl N1 of INH.
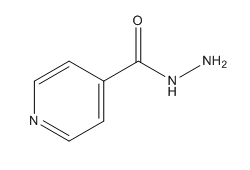
Chemical Structure of Isoniazid (INH)
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.