Sandbox Reserved 1087
From Proteopedia
| This Sandbox is Reserved from 15/04/2015, through 15/06/2015 for use in the course "Protein structure, function and folding" taught by Taru Meri at the University of Helsinki. This reservation includes Sandbox Reserved 1081 through Sandbox Reserved 1090. |
To get started:
More help: Help:Editing |
|
Contents |
Introduction
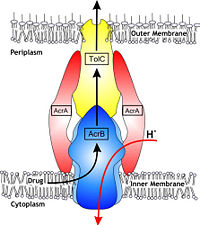
In Gram-negative bacteria, such as Escherichia coli, one of the main reasons behind bacterial multidrug resistance is resistance nodulation cell division (RND) transporters which pumps a wide range of antibiotics, dyes, bile salts and detergents out of the cell by proton motive force. Naturally, AcrB is speculated to function by pumping out of bile salts and their derivatives from the natural habitat of E. coli. for their survival with the presence of the high concentrations of these detergents. AcrB is the major RND transporter in E. coli. Three proteins form the tripartite multidrug efflux system that pumps the drugs out from the cell. The AcrB transporter, the inner membrane component of the system, cooperates with two other proteins: membrane fusion protein AcrA and an outer membrane channel TolC. The structure of the complex indicates that the drugs are transported out of the cell in a three-step procedure. [1, 2, 3]
This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Structure
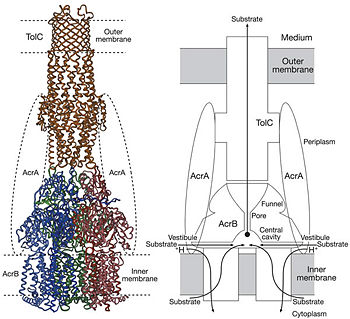
The tripartite multidrug efflux system contains three components: AcrB, the inner membrane component, AcrA, the membrane fusion protein and TolC, the outer membrane channel. The TolC component is connected to outer membrane and AcrB is connected to the inner membrane. The AcrA connects TolC and AcrB together (Fig 1.). In the AcrB component there is three domains: TolC docking domain, pore domain and the transmembrane domain. The AcrB consists of 1 049 amino acids and the three protomers of AcrB are organized as a homo (Fig. 2). The appearance of the trimer is of a jellyfish. The N- and C-terminal half’s show similar structural architecture and indicates at early gene duplication events. The trimer, formed by AcrB monomers, appears to be stabilized by the intermonomer connecting loops. [1, 2]
Transmembrane domain structure
Twelve α-helices of each promoter forms the transmembrane domain. Six α-helices in the N-terminal and the six in C-terminal are arranged symmetrically. These helices are long and they reach outside the cytoplasmic surface of the membrane. The membrane domain contains an additional extra-membrane a-helix (Ia) located between and attached to the cytoplasmic membrane surface (Fig. 3a and b). Amino acid residues 860-868 (TM8) are in a disordered state. Between and TM7 locates a groove within the transmembrane domain of each promoter. Through the disordered region of the top of TM8, the groove is connected with the cavity. This domain contains three functionally critical residues: Asp407, Asp480 and Lys940. When these are mutated the whole complex loses its drug resistance.
Pore domain structure
The pore domain consists of subdomains , , and . These subdomains have a characteristic structural motif: two b-strand–a-helix–b-strand motifs are directly repeated and sandwiched with each other. This motif forms a structure in which two a-helices are located on a four-stranded antiparallel b-sheet. Three α-helices forms a pore in the middle of the structure. The headpiece of the structure (the TolC docking domain) forms a funnel like structure in the middle of the domain. The pore connects with the bottom of the funnel (Fig 2B.). The α-helices that forms the pore are from the PN1 subunit (Fig. 2C). Between PN2 and PC2, there are vestibules open at the side of the headpiece into the periplasm. They have access to a cavity at the bottom of the pore. Analysis with the AcrA has suggested that PC1 and PC2 subdomains has a role in attaching the AcrA to the complex. Analyses has suggested that C-terminal domain residues 290-357 has a main role in interacting with AcrA. [1, 2]
TolC docking domain structure
The TolC docking domain has two subdomains: and . The subdomains contains a four-stranded mixed b-sheet. The domain forms a funnel-like structure that has a similar diameter as the bottom of TolC. The TolC docking domain of AcrB and the bottom of TolC fits well with each other for connecting. [1]
Function
Possible mechanism of transport function has been postulated. ArcB can cooperate with TolC in the TolC docking domain forming a direct pathway from the cytoplasm to the extracellular milieu. The end of the headpiece is the narrow funnel connected with the pore, whose end is connected with the extramembrane part of the central membrane hole, namely, the cavity. There might be two pathways for the substrate translocation to the cavity. One is the groove located between TM8 and TM7 in the transmembrane domain of each promoter (Fig 1C). The other is the vestibules which are open at the side of the headpiece between PN2 and PC2 of the pore domains. The substrates located in the inner leaflet of the membrane and cytoplasm would get access to the cavity through the transmembrane groove, while the substrates which are on the outer space or in the outer leaflet of the membrane are more likely to be transported to the cavity through the vestibules. Studies have been done on the substrate binding and specificity and the periplasmic part of the tripartite efflux system is found important to the substrate specificity. In the study on antibiotics to AcrB, Phe 386 (TM3) was reported as one the main hydrophobic contacts. However, currently, the theoretical explanation of the wide variety of substrates is still lacking. Proton-motive force has been proposed to be coupled with the tripartite efflux system. It is the transmembrane domain where binding and release of protons happen. Certain key residues had been identified as crucial to the protons translocation. They are the residues Lys940 () and Asp407 and 408 (TM4) harbored in and TM10 in each protomer. When the ion pairs between them are disrupted due to the transient protonation of residues mentioned above, there may be conformational change of TM4 and TM10. Through possible remote conformational coupling, the conformational change of TM4 and TM10 may induce the opening of the pore.
Relevance
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644