Function
Cellular effects
The autophagy related proteins (Atg) are proteins which purpose is to help the parasite to survive and develop. Atg8 and Atg3 are both involved in the autophagy process, by creating an autophagosome and then making it grow. The autophagosome can be compared to a vacuole and absorbs the components of the cytoplasm (in a non specific way). When the growing phase (sporozoite) of the cell is over,in the case of Plasmodium falciparum, the autophagy is launched by the cell in order to become erythrocytic-infective. This is the time when the cell starts its viral activity. Lysosomes then merge with the autophagosme to form the autolysosome. Once this process is done, the autolysosome can complete its purpose : recycle the its content. Infact, all the molecules inside are degradated and their components (like aminoacids) are reused in order to synthetise viral proteins.
Molecular effects
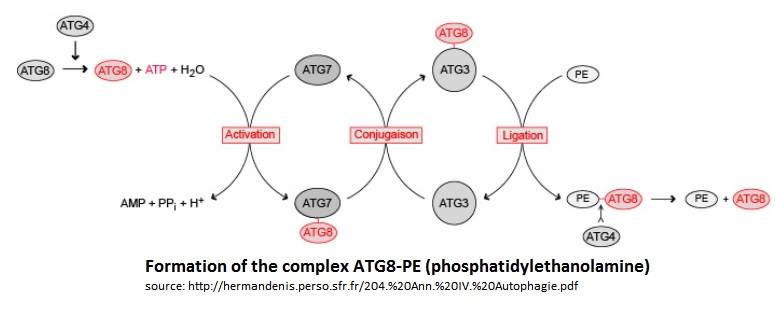
In the Atg8 system, there are several steps until Atg8 is activated. Firstly, the cysteine protease Atg4 cleaves the last residue of the C-terminal domain of Atg8 by consuming 1 molecule of ATP and H20. Then, the Atg7 (E1-like enzyme) activates the C-terminal glycine exposed and therefore forms an intermediate Atg8-Atg7 by creating a thioester bond. Atg3 (E2-like enzyme) then replaces Atg7 in the complex to form an Atg8-Atg3 thioester intermediate. Phosphatidylethanolamine (PE) then links to the N-terminal domain of Atg8. This final complex (Atg8-Atg3-PE) serves as an intermediate for membrane tethering and hemifusion of the autophagosome. This complex is primordial for the elongation of the autophagosome. Notice that Atg8-Atg3-PE is the active complex, but Atg8-PE can also be sufficient to do this task (like this is showed on the scheme below).
Disease
Malaria is an infectious and lethal disease transmitted by Anopheles mosquitos, leading to the apparition of fever, vomiting, fatigue or even coma. If this disease is not well treated, the symptoms could return even months after and can infect humans as well as other animals.
The parasit, a plasmodium type (Plasmodium falciparum for humans), introduced by the female mosquito, will infect the hepatic cells then the victim’s blood in order to destroy the red blood corpuscule.
Relevance
Structural highlights
Atg3 protein
Atg3 is a protein composed of 314 aminoacids with an alpha/beta-fold with a core region topologically similar to E2 enzymes . The core region has two regions:
→ the first region has 80 residues and has a random coil structure in solution, this region is responsible for the Atg7 interaction which is an E1-like enzyme.
→ The second is an alpha-helical structure which protrudes from the core region. This alpha-helical structure is responsible for binding Atg8.
Moreover some researches indicates that the catalytic cysteine of Atg3 is a possible binding site for a phosphate of phosphatidylethanolamine.
Atg8 protein
Atg8 is a protein of 117 aminoacids with a molecular wieght of 13,6kDa. This molecule is composed of 5- stranded β-sheet. Those β-sheet are enclosed by two α-helices on each sides. This conformation leaves accessible a conserved GABARAP domain, this protein has been originally identified as a binding partner of a GABAA receptor subunit. GABARAP. Even if the sequences between Atg8 and ubiquitin are not similars, the crystal structure reveals a conserved ubiquitine-like fold. Atg8 belongs to the ATG family but it differs from the other members of the family because the α2 helix-terminating proline 26 was substituted by a lysine.
Atg8/Atg3 Complex
Atg3 and Atg8 interact through a thioester bond between the Cys-288 of Atg3 and the C-terminal Glycine of Atg8.

