Sandbox Reserved 1124
From Proteopedia
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
Structure of the human adaptor protein Grb2
|
Plus d'info dans story, disease
remanier l'intro
mettre references
Trouver des titres plus précis
mettre une photo de monomère
Contents |
Introduction
Grb2 (Growth factor receptor-bound protein 2) is an adaptor protein implicated in signal transduction such as the Ras signalling pathway and can bind autophosphorylation sites of growth factor receptors. This small protein is essential for multiple cellular functions such as embryonic development and cell proliferation. Moreover, Grb2 can be found in the cytosol, the nucleus and the plasmic membrane.
History
The relevance of Grb2 has been enlightened thanks to studies on Caenorhabditis elegans. Sem-5, an homologue of Grb2, was found to be implied in the Let-60 pathway, an homologue of Ras.
DNA/RNA
The gene which codes the Grb2 protein is located on the seventeenth chromosome. It is composed of five exons, ranging from 78 to 186 bp, and four introns from 1 to 7 kb. It is transcribed into 2 mRNA arising from alternative splicing. Thus, there are two protein isoforms. The RNA coding for the second isoform has lost the exon of the 3' coding region, thus this isoform lacks the residues from 59 to 100 in the mature Grb2. In fact, it is a deletion in the amino-terminal part of the SH2 domain. Therefore, the function is modified because this domain cannot bind the phosphorylated tyrosine.[1]
Structure
Grb2 is a small protein of 217 residues with a molecular mass of about 25,206 Da and composed of three remarkable domains : a single SH2 (Src Homology 2) domain (60 to 152 pdb) flanked by two conserved SH3 domains (respectively 1 to 58 and 156 to 215 pdb)[1]. The two SH3 domains bind proline-rich regions of other proteins and enable the interaction with the Sos protein (Son of Sevenless, guanine nucleotide exchange factor). Moreover it has no catalytic domain. The Grb2 protein can exist in two states : monomeric or dimeric. However, only the monomeric Grb2 conformation is able to bind SOS protein and regulate MAP kinases. In this case, the dimeric Grb2 plays the role of an inhibitor. In fact, the dimer dissociation allows the phosphorylation of Grb2 160 tyrosine and the bond of SH2 domain with phosphorylated tyrosines. To conclude, the switch between these two conformations controls the MAP kinase activity.
The SH2 domain
This domain is very essential for the function of Grb2. In fact, of Grb2 enables the interaction with receptors, scaffold proteins, tyrosine kinases but also with other adaptor proteins. Indeed, Shc is an intermediate between some receptors and Grb2.
The central SH2 domain binds growth factor receptors (EGFR or PDGFR) or scaffold proteins. It interacts preferentially with a tyrosine phosphorylated sequence with the following motif: pY-X-N-X (X is a hydrophobic residue).[2]. Other non-receptor tyrosine kinases have also this motif and interact with Grb2 SH2 domain, such as BCR-Abl, focal adhesion kinase, insulin receptor substrate 1 and PTPN11.
The SH2 domain encompasses 8 beta strands ( ; ; ; ; ; ; ; ) and 2 alpha helices ( and )[3]. The βB, βC and βD strands compose a three-stranded antiparallel β-sheet and the 2 α-helices are positioned on both sides. Moreover, the short parallel βA and βG strands extend the central β-sheet. There are also βD', βE and βF strands which are smaller β-sheet-like structure. [4]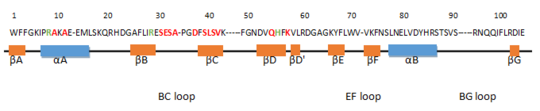
The amino acid in red are residues which are responsible for forming the phosphopeptide binding pocket. In green, these are residues which can bind to the negatively charged phosphorylated tyrosine residues of the peptide. This domain is very essential for the function of Grb2.
The N-Terminal SH3 domain
plays the main role in the interaction with the SOS protein. It binds a proline-rich motif PxxP of the C-Terminal domain of SOS[6] and this binding region in SOS has the shape of a Polyprolin II helix.
The N-terminal SH3 domain encompasses two three-stranded antiparallel β-sheets, one strand crosses the two sheets. This confers a barrel-like structure upon the domain. The first sheet contains the 3 following strands: S1 (), S2 () and S6 (). The second sheet contains the strands S3 (), S4 () and S5 (). The structure of this SH3 domain is stabilized by a high number of hydrophobic residues, which form the centre of the protein.[6]
The C-terminal SH3 domain
goes from the amino acid 156 to 215. The role of this domain is little known. But it has been shown that, for a stable complex formation, the C-Terminal SH3 domain has to recognize a 13 residues sequence with the following motif: P-x-x-x-R-x-x-K-P. Sos contains this sequence, as well as Gab1, which also binds to Grb2. The interaction of Grb2 with Gab1 has been demonstrated with precipitation experiments. ref
In the Ras pathway, the Ct SH3 domain improves the overall stability of the Grb2-SOS complex.[7]
The determination of the crystallographic structure enabled the observation of the junction between the SH3 and SH2 domains. It allows the two adjacent faces of the SH3 domains to be closer. This association is strenghtened by Van der Waals interaction. Nevertheless, the proline-rich peptides can still bind to SH3 domains. And when the SH2 domain of Grb2 binds to a receptor, the ability of the SH3 domains to interact with Sos motifs does not change.
Function
MAP kinases pathway
In the cytosol, Grb2 is bound to the guanine nucleotide exchange factor SOS-1 via its SH3 domain. Then, this complexe is recruited to the plasmic membrane to be close the Ras protein. To help Grb2 binds the phosphorylated tyrosines of the EGFR via its two SH2 domains. The Ras protein is a small GTPase which is in an inactive state when it is bound to GDP. However, the exchange of GDP for GTP actives it allowing the bond and the activation of Raf 1, a serine/threonine protein kinase. A cascade of kinase phosphorylation is then initiated. Indeed, Raf 1 phosphorylates MEK1 or MEK 2 which in turn phosphorylate ERK1 or ERK2. Finally, these MAP kinases allow the translocation of transcription factors to the nucleus and their phosphorylation. Such as STAT 1 or Elk-1.[1] Moreover, the SH2 domain recognizes the C-terminal domain of FAK (Focal Adhesion Kinase) called the focal adhesion targeting region (FAT) when its tyrosine 925 on the first helix is phosphorylated. But, this tyrosine contains the pY925 motif and is not a β-turn structure. Thus, an adaptation of this tyrosine on the SH2 domain is possible. To conclude, the interaction between Grb2 and FAK leads to the activation of the Ras-MAPK pathway.
PI3K/AKT pathway
In epithelial cells, an adaptator called Gab1 binds the carboxyl terminal SH3 domain of Grb2. This causes the activation of PI3K/AKT pathway.[1]
T cells
When TCR are stimulated, the membrane protein called p36-38 is phosphorylated in the T cells. Then, the phosphorylated tyrosines bind the SH2 domains of Grb2 whereas the SH3 domains are bound to Vav proteins. These interactions allow the T cell proliferation, the calcium flux in these cells and the MAP kinase activation.[1]
Disease
Grb2 is an intermediate protein and recruits signalling molecules to form complexes. These signalling complexes cause cellular responses like cellular proliferation or invasion which can have an impact in cancer. Grb2 can also occur in many other stages of the cancer progression : it can lead to tumorigenesis.[8] Moreover, the phosphorylation of the 160 tyrosine on Grb2 has been observed in many human cancers such as prostate, colon or breast cancers and the switch between the dimeric and monomeric conformations regulates the cancer progression.
Several mutations in the SH2 domain can cause human diseases such as Noonan syndrome or basal cell carcinoma. For example, it can have a suppression of phosphotyrosine dependent interactions due to a mutation of the arginin residue at position 5 of the βB strand in the SH2 domain.[5] This suppression can lead to a non-functioning signalling pathway.
</StructureSection>
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Gagani Athauda, Donald P Bottaro Atlas of Genetics and Cytogenetics in Oncology and Haematology (2007)[1]
- ↑ Higo K, Ikura T, Oda M, Morii H, Takahashi J, Abe R, Ito N. High resolution crystal structure of the Grb2 SH2 domain with a phosphopeptide derived from CD28. PLoS One. 2013 Sep 30;8(9):e74482. doi: 10.1371/journal.pone.0074482. eCollection, 2013. PMID:24098653 doi:http://dx.doi.org/10.1371/journal.pone.0074482
- ↑ UniProtKB P62993 Human
- ↑ Ogura K, Shiga T, Yokochi M, Yuzawa S, Burke TR Jr, Inagaki F. Solution structure of the Grb2 SH2 domain complexed with a high-affinity inhibitor. J Biomol NMR. 2008 Nov;42(3):197-207. doi: 10.1007/s10858-008-9272-0. Epub 2008, Oct 2. PMID:18830565 doi:http://dx.doi.org/10.1007/s10858-008-9272-0
- ↑ 5.0 5.1 Kousik Kundu In Silico Prediction of Modular Domain-Peptide Interactions (2015) [2]
- ↑ 6.0 6.1 doi: https://dx.doi.org/10.1038/nsb1294-898
- ↑ doi: https://dx.doi.org/http
- ↑ Giubellino A, Burke TR Jr, Bottaro DP. Grb2 signaling in cell motility and cancer. Expert Opin Ther Targets. 2008 Aug;12(8):1021-33. doi: 10.1517/14728222.12.8.1021, . PMID:18620523 doi:http://dx.doi.org/10.1517/14728222.12.8.1021
