This is a default text for your page Nos1. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Function
Disease
Relevance
Structural highlights
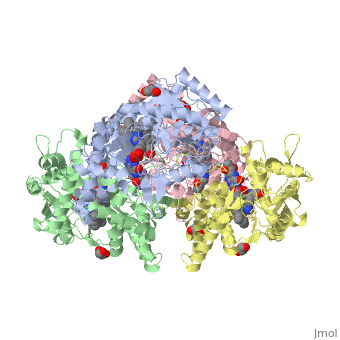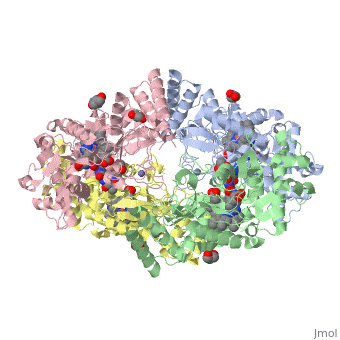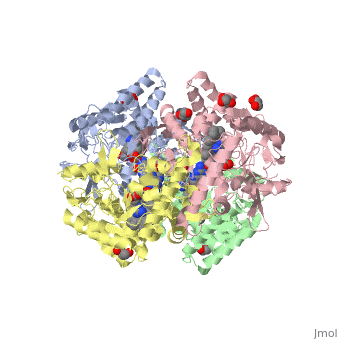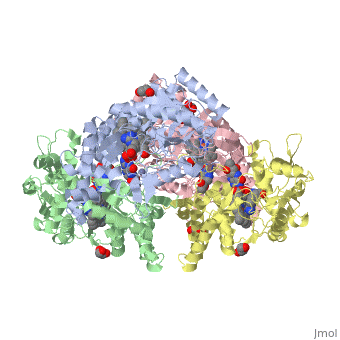
Nos exists as a homodimer. This homodimer consists of two regions. One region is an N-terminal oxygenase domain and the other is a C-terminal reductase. The N-terminal oxygenase domain is an extended beta sheet cage with binding sites for heme and pterin. Nos has a N-terminal catalytic domain with a heme active site. Nos 1 also has a cofactor site nearby for tetrahydrobiopterin (H4B). Nos has a C-terminal reductase domain containing FMN, FAD and NADPH binding sites. Electrons are passed from FAD to FMN and then to the heme. This electron flow is controlled by the binding of CaM and Ca2+ in the linker region between the two major domains. The linker between the oxygenase and reductase domains contains a calmodulin-binding sequence. Nos1 is found in neuronal tissue.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.