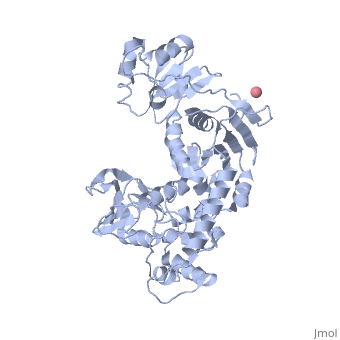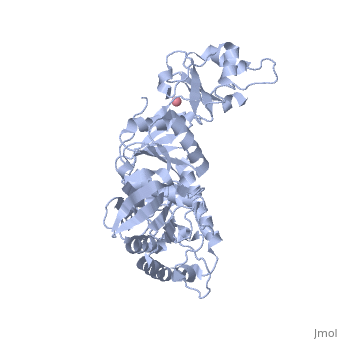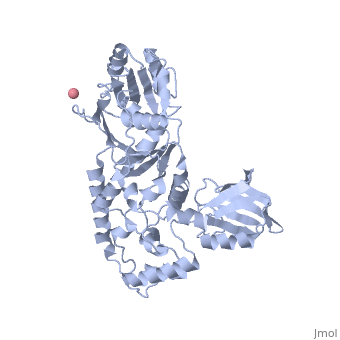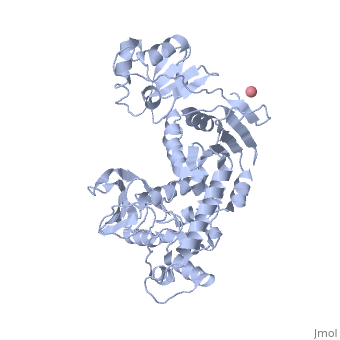
Crystal Structure of Transcription-repair coupling factor
2eyq
Function
Transcription-repair coupling factor (TRCF) or Mfd enables the coupling of these processes in bacteria and humans. The TRCF has ATPase activity.
Size exclusion chromatogaphy indicates that the protein
Structural highlights
an N→C rainbow similar to Figure 1A of the article describing the structure. .
The domains in the structure of Mfd:
similar to Figure 1B and Figure 2 of the article describing the structure.
Figure 1B and Figure 2 of the article describing the structure. .
[Note: the following view generates a substantial surface which may take several minutes to calculate. Use the one above as an alternative unless you are willing to spend the time.] Figure 1B.
displayed as in
Figure 3A of the article describing the structure.
are highlighted in red. See figure 3B.
displayed similar to
Figure 3B of the article describing the structure.
indicated similar to Figure 3C of the article describing the structure.
3D Structures of Transcription-repair coupling factor
Updated on 08-December-2020
2eyq, 2b2n – EcTRCF – Escherichia coli
3hjh – EcTRCF residues 1-470
6yhz - EcTRCF residues 472-547 – NMR
4dfc – EcTRCF D2 domain 127-213 + UvrABC system protein A
6xeo – EcTRCF + DNA – Cryo EM
3mlq – TtTRCF RNA polymerase interacting domain + DNA-directed RNA polymerase subunit β - Thermus thermophilus
6m6a – TtTRCF + RNA polymerase – Cryo EM
6m6b – TtTRCF + RNA polymerase + ATP-γ-S – Cryo EM
2qsr – TRCF C terminal – Streptococcus pneumoni
6ac6, 6aca, 6ac8 – MsTRCF – Mycobacterium smegmatis
6acx – MsTRCF + ADP