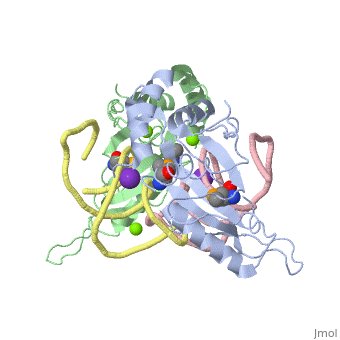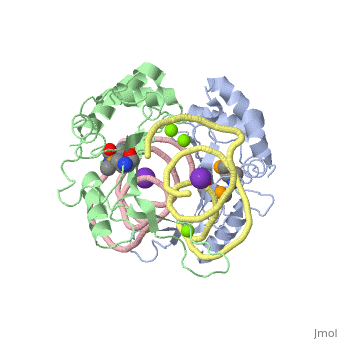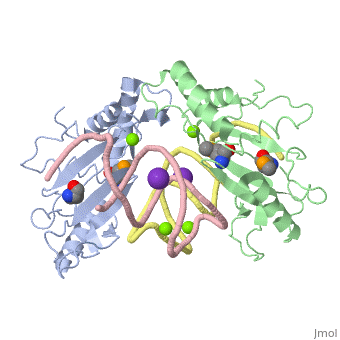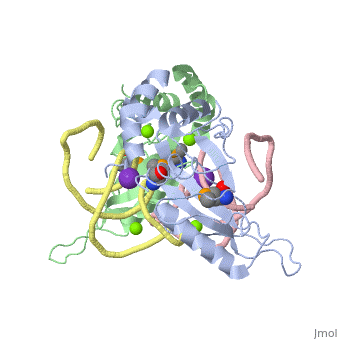
The ribosome is an essential component of all living cells. It is responsible for translation of proteins using messenger RNA (mRNA) and transfer RNA (tRNA). Ribosomes are composed of both proteins and RNA known as ribosomal RNA (rRNA). There are three functional sites found within the ribosome that are essential for translation: the A, P, and E-sites. The A-site is responsible for recruiting the tRNA and the E-site is where the tRNA leaves from once the protein is removed in the P-site. The site of interest for this sandbox is the P-site, where the protein is removed from the tRNA and added to the growing chain of amino acids. Its primary component is the L5/5S rRNA complex. This complex has been proven to be an essential component to both the structure and the function of the ribosome.[1]
Function
The primary function of the L5 protein is to anchor the peptide-linked tRNA to the P-site for the transfer of the peptide to the growing chain.[2]
The L5 protein is also responsible for the assembly of the ribosome. In ribosomes that fail to synthesize the L5 protein, the ribosome is unable to connect the large and small units together. Without this protein, the cell would only be able to divide a select few times. [3]
The 5S rRNA is responsible for enhancing protein synthesis. This is done by stabilizing the structure of the ribosome.[4] 5S rRNA has also been noted to be a major component in tumor suppression.[5]PMID:27528756</ref>
Structural highlights
The makes up the main function and structural component of the large ribosomal sub-unit.
is one of many residues within the L5 protein that have a crucial role in the binding affinity of 5S rRNA.[6] Mutations on these residues have been shown to drastically decrease they probability of the 5S rRNA binding to the L5 protein. Residues like directly influence the binding ability of 5S rRNA. Mutations in these residues result it a less stable interaction between the 5S rRNA and L5 protein. [7]
The 5S rRNA has many that contribute to both its structure and function. These residues include C47, U48, C37, C38, A34, and G44. The structural role comes from stacking interactions that occur from the binding of these residues.[8]