User:Harish Srinivas
From Proteopedia
Contents |
Introduction
Sphingolipids together with glycerol-based phospholipids are major structural components of cell membranes. In response to various extracellular stimuli, including growth factors, inflammatory cytokines, antigens, and agonists of some GPCRs, the sphingolipids can be metabolized into potent mediators, such as Sphingosine-1-phosphate (S1P). This sphingolipid has emerged as an important signaling mediator participating in the regulation of multiple physiological and pathological processes taking place in cancer, cardiovascular diseases, wound healing, atherosclerosis and asthma but also is important in pathological conditions such as inflammation and stress. It can also trigger a range of biological effects such as cell migration, differentiation, apoptosis, immunity, proliferation and angiogenesis. The functioning of S1P receptors in the maintenance and modulation of the activity of the biological barrier is of the profound biological importance and has many therapeutic implications including treatment of multiple sclerosis, prevention of the transplant rejection and probably the adult respiratory distress syndrome as well.
|
Structural Features
Transmembrane region
One cluster of core contacts links transmembrane (TM) I, II, and VII, cluster 1 consists of individual interaction groups. The second cluster of interactions links TM II, III, and IV through a series of four interaction groups and a frequently observed interaction between position interact through a hydrogen bond between an Asn or Ser and the indole nitrogen of a Trp. A third cluster of conserved contacts links together TM helices III, VI, and VII in the vicinity of the S1P1 receptor ligand binding pocket. These positions maintain important contacts between TM VI and TM III through two side chain-mediated interactions. Finally, cluster number four consists of three interactions that constrain TM V relative to TM III and one interaction between TM V and TM VI.
Exrtra-cellular region
The extracellular region for all GPCRs consists of three loops: ECL1 between TM helices II and III, ECL2 between TM helices IV and V, and ECL3 between TM helices VI and VII. Optionally, there is a structured N-terminus that interacts with the ECLs. In the case of S1P1 receptor the structured N-terminus occludes the binding pocket, in the antagonist-bound state, cutting off access to the extracellular environment. One possible role for this structured N-terminus is that it is a feature of the S1P1 receptor structure in general and its presence implies the ligand does not access the binding pocket from the extracellular space directly. Instead, it is possible that the ligand gains access to the binding pocket through the lipid membrane where there is an enlarged gap between TM I and TM VI. This gap is larger in the S1P1 receptor than other class A GPCRs largely due to a shift in the position of the extracellular end of TM I away from TM VII in the S1P1 receptor.
Ligand binding region
The S1P1 receptor provides 18 residues from the transmembrane region for interactions with the ML056 antagonist along with three additional residues from ECL2 and two from the N-terminus. ML056 lies in an amphipathic pocket where the head group phosphonate interactions are largely polar in nature and the alkyl chain tail interactions are largely hydrophobic as would be expected. The polar interactions observed for ML056 largely confirm mutagenesis data establishing the importance of Arg120 and Glu121 which were identified as important residues for supplying interactions with the zwitterionic sphingosine head group. In addition, the phosphonate head group of ML056 is surrounded by a ring of positively charged and polar residues contributed by TM helices III and VII, ECL2, and the N-terminal capping helix. Together these residues form a pocket that provides charge complementarity and high-affinity interactions to the phosphate group of the sphingolipids. A feature of ML056 is a primary amine located in the beta position relative to the phosphonate group. This primary amine is likely protonated and charged at physiological pH, thus enhancing its interactions with Glu121 through salt bridge formation. In addition to Glu121, Asn101 and Tyr98 provide hydrogen bonding interactions with the primary amine and amide linkage of ML056, respectively. The phenyl aryl tail of ML056 inserts into a hydrophobic pocket consisting of residues from TM helices III, V, VI, and VII, as well as ECL2. The pocket is lined with short aliphatic residues that define the shape and hydrophobicity of the pocket and four aromatic residues that provide the potential for specific interactions.
Lipid Receptor S1P1 Activation Scheme
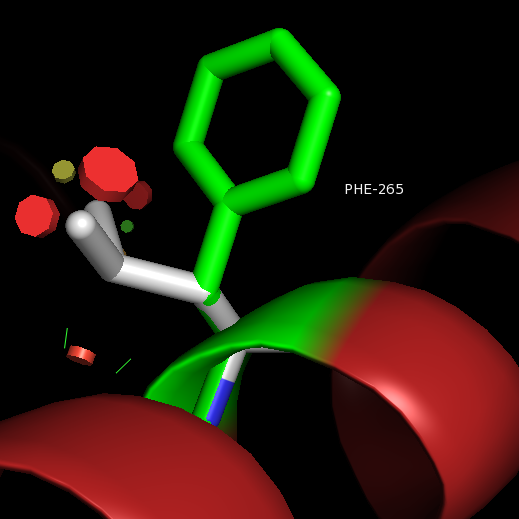
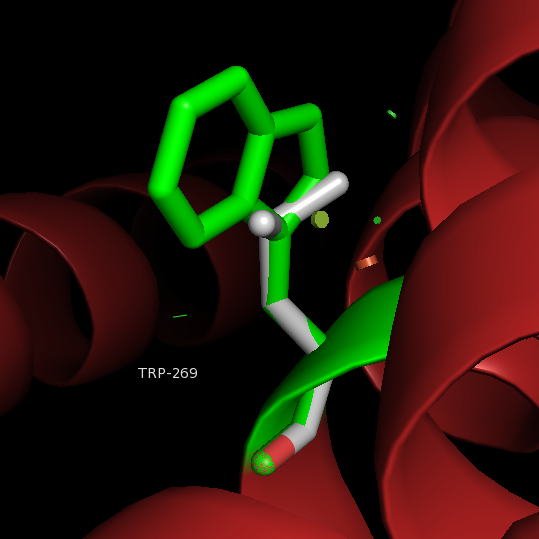
After binding of agonist S1P to the binding site of S1P1, the movement of acyl tail of S1P leads to the flipping of W269 (step 1). Such rotameric change alters the conformation of side chain of F265 which is located next to W269 in the same helix TM6 (step 2). These residues form a core of a transmission switch which involves rearrangement of centrally located residues including N63, D91, S304 and N307 They facilitate a redirected flow of water molecules inside a receptor (step 3). The influx of water molecules at intracellular part of the receptor leads to limited motions of cytoplasmic ends of TM helices, with the largest movement associated with TM7 (step 4), which is a prerequisite for larger motions of the cytoplasmic parts of transmembrane helices. These movements lead to opening the protein structure to make room for binding a G protein.
Antagonism versus agonism
Ligands that bind to S1P1 function either as an agonist or antagonist based on minor differences in their structures, which translates to activating/deactivating shifts in critical receptor residues. Overall, the activating effect of a ligand on S1P1 is dependent on the length and position of its acyl chain in the ligand-binding pocket with respect to its amine and phosphate/phosphonate headgroup. Interestingly, the S1P1 structure shows that the antagonist ML056 is entirely in its meta conformation. Hanson and colleagues observed that switching the conformation pattern from meta to para switches the ligand effect from antagonism to agonism. In addition to the conformation of ligand, the volume of the ligand-binding pocket that is occupied also plays a critical role in receptor activation. An increase in length of the acyl chain of a ligand effectively switches its effect from antagonism to agonism. ML056 variants extending the acyl chain up to three additional methylenes can be accommodated in the binding pocket with no significant side chain shifts. However, once the total number of methylenes in the acyl chain reaches 10 (i.e. ML056 extended by four methylenes), the ligand reaches the point at which significant positional shifts are needed in the binding pocket residues to accommodate the ligand.
Mutagenesis
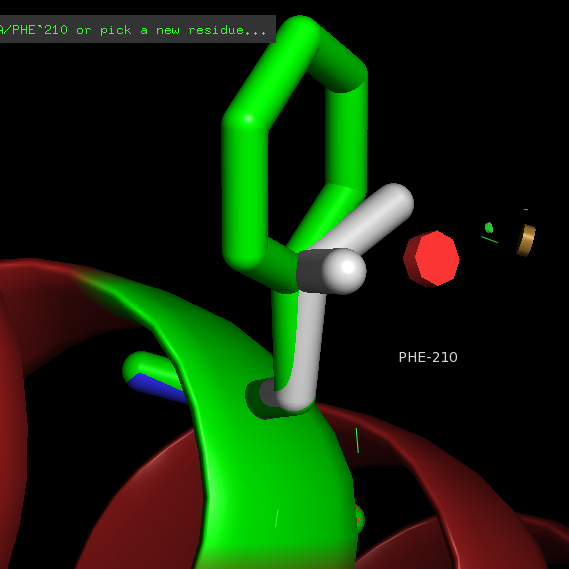
F → L 210 Impairs sphingosine 1-phosphate binding and signaling.
F → L 265 Impairs sphingosine 1-phosphate binding and signaling.
W → F 269 Impairs sphingosine 1-phosphate binding and signaling.
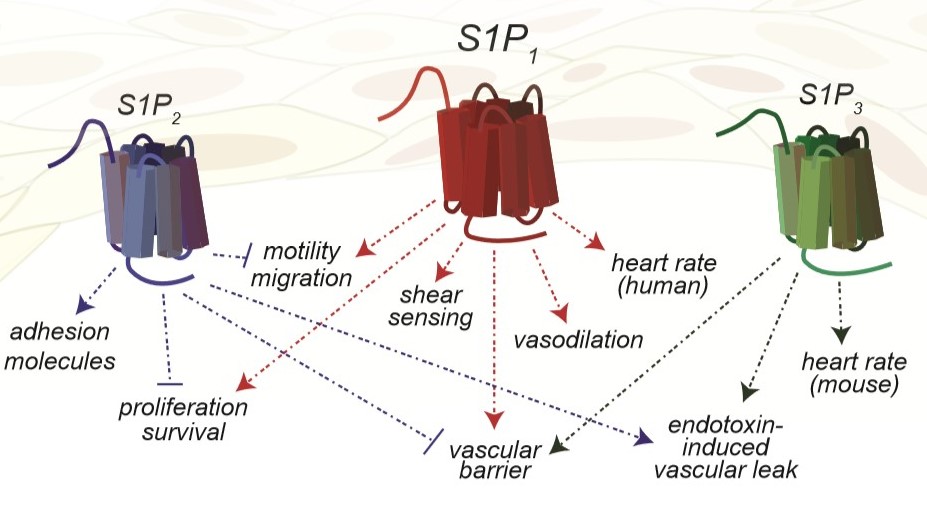
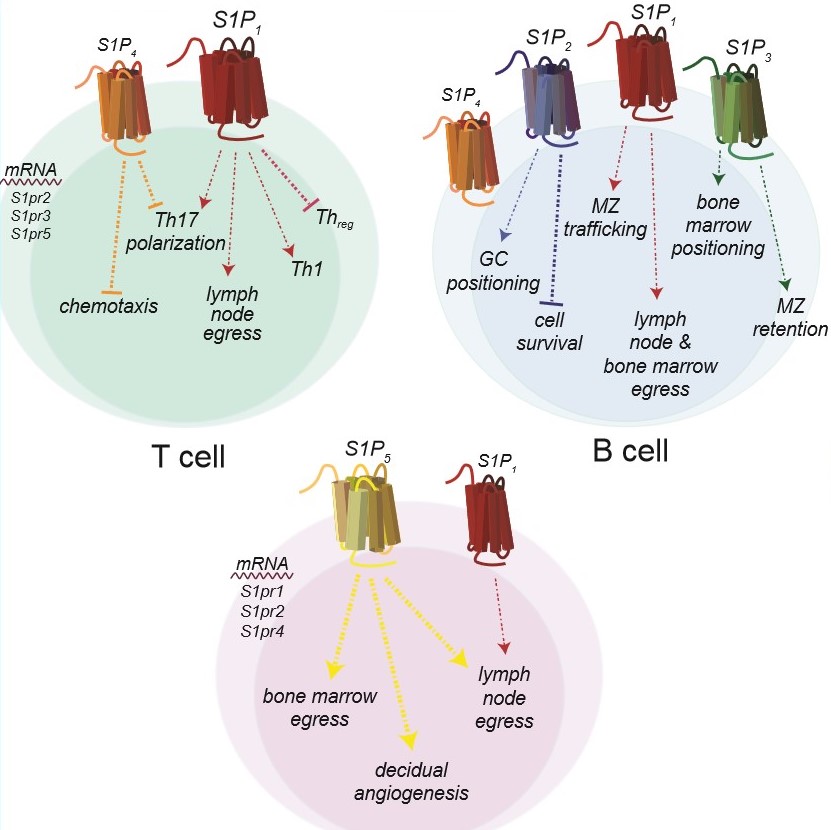
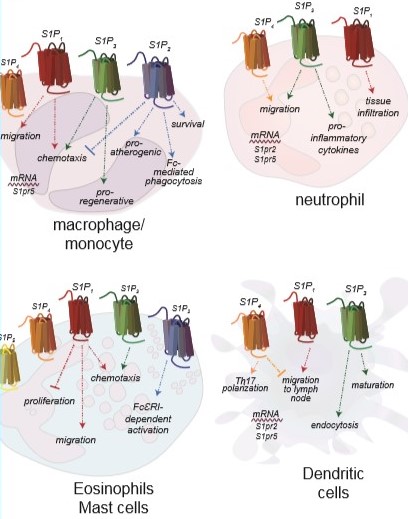
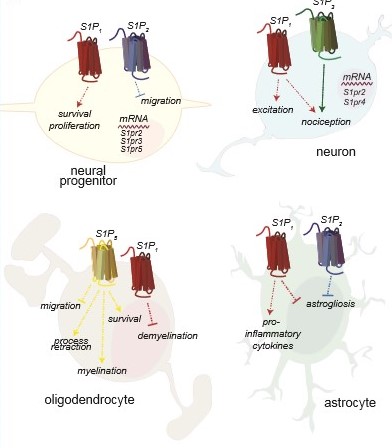
Biology of sphingosine 1-phosphate receptors
Vascular and Lymphatic systems
Immune systems
Nervous systems
References
1.An update on the biology of sphingosine 1-phosphate receptors Victoria A. Blaho and Timothy Hla* Center for Vascular Biology, Department of Pathology and Laboratory Medicine, Weill Cornell Medical College, 1300 York Ave., New York, NY 10065
2.Lipid Receptor S1P1 Activation Scheme Concluded from Microsecond All-Atom Molecular Dynamics Simulations
Shuguang Yuan1,2,3*, Rongliang Wu1, Dorota Latek1, Bartosz Trzaskowski4, Slawomir Filipek4*
1International Institute of Molecular and Cell Biology in Warsaw, Warsaw, Poland, 2Laboratory of Physical Chemistry of Polymers and Membranes, E ´cole Polytechnique Fe ´de ´rale de Lausanne, SB ISIC LCPPM, Lausanne, Switzerland, 3Nencki Institute of Experimental Biology, Polish Academy of Sciences, Warsaw, Poland, 4Faculty of Chemistry, University of Warsaw, Warsaw, Poland
3.Sphingosine-1-phosphate metabolism: A structural perspective
Michael J. Pulkoski-Gross1, Jane C. Donaldson2,3, and Lina M. Obeid2,3,4 1Department of Pharmacological Sciences, Stony Brook University, Stony Brook, NY, USA
2Department of Medicine, Stony Brook University, Stony Brook, NY, USA
3Stony Brook Cancer Center, Stony Brook, NY, USA
4Northport Veterans Affairs Medical Center, Northport, NY, USA
4.Structural Biology of the S1P1 Receptor
Michael A. Hanson and Robert Peach
5.Structure of the First Sphingosine 1-Phosphate Receptor
Abby L. Parrill1,*, Santiago Lima2, and Sarah Spiegel2,* 1Department of Chemistry, The University of Memphis, 213 Smith Chemistry Building, Memphis, TN 38152, USA 2Department of Biochemistry and Molecular Biology, Virginia Commonwealth University (VCU) School of Medicine, 1101 East Marshall Street, Richmond, VA 23298, USA
6.A Single Amino Acid Determines Lysophospholipid Specificity of the S1P1 (EDG1) and LPA1 (EDG2) Phospholipid Growth Factor Receptors*
Received for publication, July 31, 2001, and in revised form, October 15, 2001 Published, JBC Papers in Press, October 16, 2001, DOI 10.1074/jbc.M107301200
De-an Wang‡§, Zsolt Lorincz‡§, Debra L. Bautista¶�, Karoly Liliom‡**, Gabor Tigyi‡ ‡‡, and Abby L. Parrill¶‡‡§§ From the ‡Department of Physiology, University of Tennessee Health Sciences Center Memphis, Memphis, Tennessee 38163 and the ¶Department of Chemistry and Computational Research on Materials Institute, The University of Memphis, Memphis, Tennessee 38152
7.Defective sphingosine-1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation
Christopher S. Garris1,2,*, Linfeng Wu3,*, Swati Acharya4, Ahmet Arac5, Victoria A. Blaho6, Yingxiang Huang1, Byoung San Moon1, Robert C. Axtell1, Peggy P. Ho1, Gary K. Steinberg5, David B. Lewis4, Raymond A. Sobel7, David K. Han8, Lawrence Steinman1, Michael P. Snyder3, Timothy Hla6, and May H. Han1 1Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA
2Graduate Program in Immunology, Division of Medical Sciences, Harvard Medical School, Boston, MA 02115, USA
3Department of Genetics, Stanford University School of Medicine, Stanford, CA 94305, USA
4Department of Pediatrics, Division of Allergy, Immunology and Rheumatology, Stanford, CA 94305, USA
5Department of Neurosurgery, Stanford University School of Medicine, Stanford, CA 94305, USA
6Center for Vascular Biology, Department of Pathology and Laboratory Medicine, Weill Cornell Medical College, Cornell University, New York, NY 10065, USA
7Department of Pathology, Stanford University School of Medicine, Stanford, CA 94305, USA
8Center for Vascular Biology, Department of Cell Biology, University of Connecticut Health Center, Farmington, CT 06030, USA
8.Identification of Tricyclic Agonists of Sphingosine-1-phosphate Receptor 1 (S1P1) Employing Ligand-Based Drug Design Hai-Yun Xiao,† Scott H. Watterson,† Charles M. Langevine,† Anurag S. Srivastava,† Soo S. Ko,† Yanlei Zhang,† Robert J. Cherney,† Wei-Wei Guo,† John L. Gilmore,† James E. Sheppeck, II,† Dauh-Rurng Wu,† Peng Li,† Duraisamy Ramasamy,‡ Piramanayagam Arunachalam,‡ Arvind Mathur,† Tracy L. Taylor,† David J. Shuster,† Kim W. McIntyre,† Ding-Ren Shen,† Melissa Yarde,† Mary Ellen Cvijic,† Anthony M. Marino,† Praveen V. Balimane,† Zheng Yang,† Dana M. Banas,† Georgia Cornelius,† Celia J. D’Arienzo,† Bethanne M. Warrack,† Lois Lehman-McKeeman,† Luisa M. Salter-Cid,† Jenny Xie,† Joel C. Barrish,† Percy H. Carter,† Alaric J. Dyckman,† and T. G. Murali Dhar*,† †Research and Development, Bristol-Myers Squibb Company, P.O. Box 4000, Princeton, New Jersey 08543-4000, United States ‡BMS Biocon Research Center, Bangalore 560099, India
9.S1P1-Selective In Vivo-Active Agonists from HighThroughput Screening: Off-the-Shelf Chemical Probes of Receptor Interactions, Signaling, and Fate
Euijung Jo,1 M. Germana Sanna,1, Pedro J. Gonzalez-Cabrera, 1 Shobha Thangada,2, Gabor Tigyi,3 Daniel A. Osborne,4 Timothy Hla,2, Abby L. Parrill, 4 and Hugh Rosen1,*, 1Department of Immunology, The Scripps Research Institute, 10550 North Torrey Pines Road, ICND 118La Jolla, California 92037 2Center for Vascular Biology, University of Connecticut Health Center, Farmington, Connecticut 06030 3Department of Physiology, University of Tennessee Health Science Center, Memphis, Tennessee 38163, 4Department of Chemistry, University of Memphis, Memphis, Tennessee 38152
10.Sphingosine-1 phosphate receptor (S1p1), a critical receptor controlling human lymphocyte trafficking, is expressed in hen and human ovaries and ovarian tumors Michael J Bradaric1, Animesh Barua1,2,3, Krishna Penumatsa1, Yu Yi 1, Seby L Edassery1, Sameer Sharma2,4, Jacques S Abramowicz2, Janice M Bahr5, Judith L Luborsky1,2*