Nuclear polyadenylated RNA-binding protein
From Proteopedia
Contents |
Introduction
|
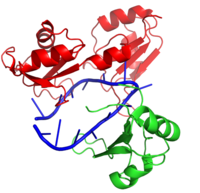
Hrp1 is a polyadenylation factor found in Saccharomyces cervisiae (yeast) [1]. This protein recognizes and binds to an RNA sequence in the 3'UTR upstream from the cleavage site called the polyadenylation enhancement element (PEE) [1]. Upon binding to the RNA, Hrp1 helps recruit additional proteins necessary for the cleavage and polyadenylation of the RNA molecule [1]. The structure of the Hrp1-PEE complex reveals the mechanism by which Hrp1 is able to recognize and bind to its specific RNA sequence at the atomic level [1].
Structure
Hrp1 is a single strand RNA-binding protein composed of two RNP-type RNA-binding domains (RBDs) arranged in tandem with a typical ßαßßαß architecture [1]. The two RBDs have similar topolgies, both containing a central antiparallel four-stranded with two α-helices running across one face [1]. The two RBDs associate to form a deep and positively charged , which constitutes the binding site for the RNA molecule [1].
Hrp1-RNA Interactions
The interface between Hrp1 and its target RNA sequence is dominated by interactions between key aromatic residues and RNA bases [1]. Only six RNA bases, an repeat, act as the PEE and form specific contacts with Hrp1 [1]. Hydrophilic residues of Hrp1 provide base specificity through hydrogen bonding [1]. Most of the key residues that interact with the RNA can be found in the ß-sheet region of Hrp1; however, loops and the interdomain linker are also essential for Hrp1-RNA recognition [1]. Perhaps the most important Hrp1-RNA interaction is the (a conserved residue) [1]. In this case, Trp168 stacks on Ade4 and forms crucial base-specific hydrogen bonds [1]. It is also worth noting that a second Hrp1 residue is critical to holding Ade4 in place, , which interacts via hydrogen bond with the N1 of Ade4 [1]. A third contributor, , also stacks with Ura7 to aid in RNA recognition and binding [1].
RBD-RBD Interactions and the Linker Region
As mentioned above, Hrp1 is composed of two RBDs. The RBDs are connected by a linker region which also contains an crucial residue for RNA binding. Ile234 holds Ade6 stacked in place with Phe162 . Experimental evidence from the NMR data [1] suggests that the two RBDs act independently until binding the PEE. Binding the PEE causes the linker region to adopt a short helical structure to rigidly hold the . Aside from the linker helix, the only interaction between the RBDs is due to between Lys231 and Asp271.
Relationship to other proteins
The RNP-type RBD is found in many proteins involved in post-transcriptional pre-mRNA processing (5' end capping, splicing, 3' end polyadenylation, and transport from the nucleus)[2].
Interaction with RNA15
RNA15 is another RNA-binding protein with a single N-terminal RNA recognition motif (RRM) [3]. RNA15 recognizes an A-rich positioning element (PE) downstream from the PEE but upstream from the 3' cleavage site [3]. The recognition of the PE by RNA15 is crucial for precise cleavage of the RNA molecule. Hrp1 and RNA15 are held together by a separate protein, RNA14 [3]. These proteins act together to anchor the polyadenylation and cleavage protein machinery relative to the cleavage site for precise 3'-end processing [3].
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 Perez-Canadillas JM. Grabbing the message: structural basis of mRNA 3'UTR recognition by Hrp1. EMBO J. 2006 Jul 12;25(13):3167-78. Epub 2006 Jun 22. PMID:16794580
- ↑ Clery A, Blatter M, Allain FH. RNA recognition motifs: boring? Not quite. Curr Opin Struct Biol. 2008 Jun;18(3):290-8. doi: 10.1016/j.sbi.2008.04.002. PMID:18515081 doi:http://dx.doi.org/10.1016/j.sbi.2008.04.002
- ↑ 3.0 3.1 3.2 3.3 Leeper TC, Qu X, Lu C, Moore C, Varani G. Novel protein-protein contacts facilitate mRNA 3'-processing signal recognition by Rna15 and Hrp1. J Mol Biol. 2010 Aug 20;401(3):334-49. Epub 2010 Jun 19. PMID:20600122 doi:10.1016/j.jmb.2010.06.032
Proteopedia Page Contributors and Editors (what is this?)
Cory A. Wuerch, Matthew Douglas Moore, Savannah Davis, Michal Harel, Jaime Prilusky