General Description
is a 97 kDa, class IA aminoacyl-tRNA synthetase (ARS) that catalyzes the ligation of leucine with tRNAleu in an ATP dependent mechanism. ARS are essential to all life as they charge amino acids onto cognate tRNAs in preparation for protein translation. This is step is a potential source of error in interpreting the genetic code as mischarged tRNAs will not be recognized during protein synthesis and could lead to nonfunctional proteins. As such, ARS have a high specificity for both tRNA and amino acids and contain an editing domain capable of hydrolyzing mischarged tRNA.
LARS is a cytoplasmic enzyme that is found as part of the multisynthetase complex in eukaryotes[1]. The multi-synthetase complex contains glutamylprolyl-tRNA synthetase (EPRS), isoleucyl (IARS), leucyl (LARS), glutaminyl (GARS), methionyl (MARS), lysyl (KARS), arginyl (RARS), and aspartyl (DARS) tRNA synthetases as well as p18, p38 and p43[2].
Multisynthetase complexes have also been seen in some archaea such as Thermococcus kodakarensis although the composition of the complex is not the same as eukaryotes[3].
LARS has been shown to be involved with the mTOR pathways as a sensor of leucine levels within the cell[4].
Disease
Mutations in LARS2, the mitochondrial version of the enzyme, have been linked to Perrault syndrome characterized by premature ovarian failure in females and progressive hearing loss in both sexes[5]
Structure
Catalytic Domain
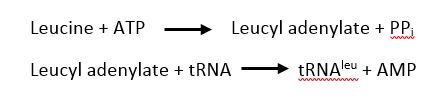
The is responsible for the two step process of charging leucine on to tRNAleu. First, ATP and leucine are bound and AMP is transfered to the backbone carboxylic acid of leucine with the release of a pyrophosphate. Second, tRNAleu is bound with the leucyl adenylate and leucine is transfered to either the 2' OH of the 3' terminal adenine with the release of AMP[6].
LARS, like all class I synthetases, is characterized by a Rossmann-fold catalytic domain with a central parallel β-sheet with α-helices on both faces. The active site catalyzes both the formation of the leucyl adenylate intermediate and the subsequent charging of leucine onto the terminal acceptor arm of the tRNA[7].
Editing Domain
The of LARS is responsible for editing mischarged tRNA and ensuring translational fidelity. The recognition of the correct tRNA has been found to be a more complex process than previously thought due to the nature of wobble bases in the codon-anticodon pair.
[8]
[9]
Anticodon Binding Domain
The is essential for the fidelity of ARSs. However, there are 6 anticodons in E. coli that correspond to leucine including AAU, CUG, and GUG, so how does the enzyme recognize so many different tRNAs? This is accomplished by recognition of the D-loop by the enzyme rather than the actual anticodon. The anticodon binding domain interacts with , 12-16 and 22-26, with U16 being essential for recognition. U16 specifically interacts with Arg718 and Lys711 within helix 4 forming hydrogen bonds.
The anticodon bindind domain is an all α helical domain made up of 8 helices in a bundle. Helices 3-5 form the tRNA recognition surface and are responsible for interaction with the D-loop of the incoming tRNA.
Zinc Binding Domain
The
Leucine Specificity Domain
The
Evolutionary Conservation
LARS is essential for protein synthesis and as such is necessary for all cellular life and present in all three kingdoms of life. Eukaryal and archaeal LARS are similar and both are structurally different from bacterial LARS. Most of the differences occur in tRNA recognition sites while the core of the catalytic and editing domains are highly conserved. The differences between bacterial and archaeal tRNA, most notably the truncated variable arm in archaea, begin to explain the structural changes that are seen in the evolution of the enzyme[10].
A shows that there is a high degree of conservation in LARS, especially within the catalytic domain. The anticodon binding domain shows a higher variation. This is to be expected as there is a larger amount of variation in codon usage, most notable between bacteria and archaea/eukarya.



