Tetrahydroprotoberberine N-methyltransferase
From Proteopedia
Structure
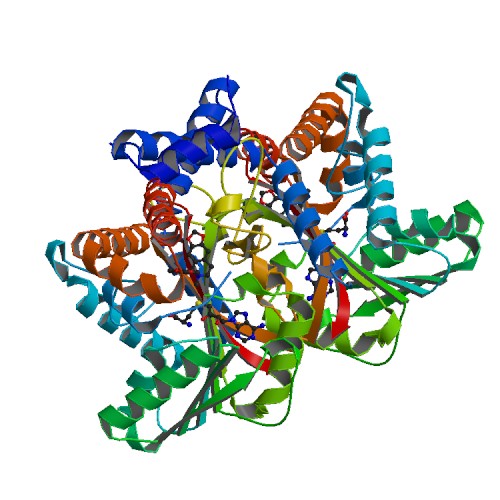
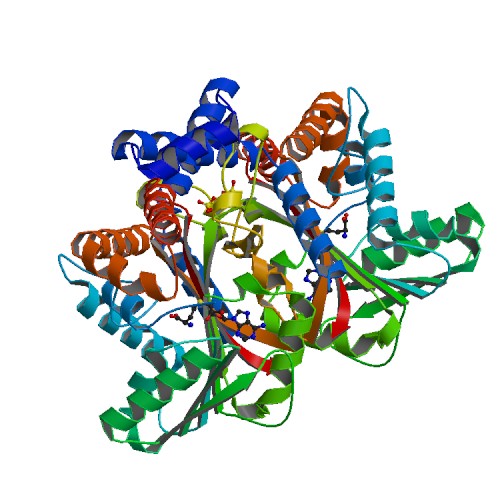
Tetrahydroprotoberbine N-methyltransferase is a protein thats dimer interface includes six salt 6 salt bridges and 8 hydrogens bonds. It is expressed in E. coli and crystallized at a pH of 7.0. The crystals were grown in the presence of SAH,SAM, and SAH+SMS. Below are the two different substrates that were in the presence of the crystallized protein. The substrate SAM is shown to the right.
FunctionThe protein being studied, Tetrahydroprotoberbine N-methyltransferase, is found in yellow horned poppy (Glaucium Flavum). The function of the protein is substate recognition as well as catalysis for the ration engineering of enyzmes for chemoenzymatic synthesis and metabolic engineering. The relative activity of about 8 substrates were tested wiht Protoberberine having the highest percentage. GfTNMT's activity depends on temperature and pH. When the enzyme's activity was at a pH of 8, dropped 10% in activity. 10% activity was also dropped when the temperature was at 30 degrees Celsius. When at 4 degrees Celsius, the activity dropped even more down to 40%. MutantsIn TMNT, three amino acid residues in the alpha14-helix form one side of the defining the BP region. The binding pocket consists of His-328(green), Ile-329(purple), and Phe-332(orange). The H328 mutation decreases in activity with stylopine and scoulerine producing a 5- and 2-fold while the activity with THP increases 2-fold. RelevanceStudying Tetrahydroprotoberbine will provide commercial application where one will gain a lot of knowledge from both the research paper and online sources. Studying this protein will allow readers to engage in the material and apply their own knowledge to better understand the study. This research will provide descriptive roles that TNMT plays such as pathway leading to the formation of different substrates including Protoberberine. [1] Structural HighlightsThis protein has a which consists of amino acids His-208, Glu-204, and Glu-207. The authors explained within the paper that other amino acids may play a role in the triad as well. They were unsure but those three were the most accurate. These amino acids play an important role in catalysis for the protein. [2] The basic of the entire protein alllows readers to visualize the different elements show in different colors. The elements shown are carbons(grey), nitrogen(blue), and oxygen(red). This protein has a which is SAM. There are of SAM that form a small catalytic pocket and surrounds the amino group and methyl donor of SAM. This catalytic pocket forms a L shape. In green Glu-204, yellow is Glu-207, red is His-208, and Tyr-81 is blue. This scene displays the active site of my the protein. In green, there is Alanine, in yellow Aspartic Acid, and in blue, Valine.
| ||||||||||||