This is a default text for your page Elizabeth M. Ratz/Sandbox 1. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction
Structure
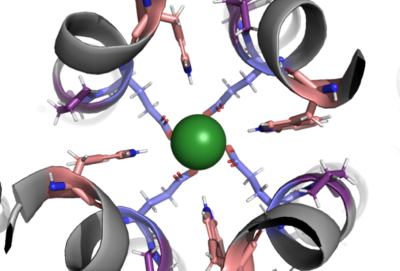
Selectivity Filter
The selectivity filter contains Glu358, Trp354, and Pro359 to allow calcium to pass through the uniporter. The carboxylate oxygen of the Glu side chains draw in the positive calcium ion. The diameter of the carboxyl ring is 5Å, allowing only a dehydrated Ca ion to bind. Trp38, which is directly next to the Glu residues, stabilizes the carbonyl side chains through hydrogen bonding and anion pi interactions. These Trp residues also form stacking interactions with Pro359, which orientate the Glu carboxyl side chains towards the middle of the pore to interact with Ca ions.
Calcium Uniporter Structure
[3]
Function
Disease
Relevance
Structural highlights
Student Contributors
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.