Background
The ABCG2 multidrug transporter is a membrane protein from the ATP-Binding Cassette (ABC) transporter family, specifically the G-subfamily. Also know as the breast cancer resistance protein (BCRP), ABCG2 has physiological roles in various tissue cells including the mammary gland and the blood-brain, blood-testis, and maternal-fetal barriers.[1] ABCG2 protects cells by exporting xenobiotic molecules out of the cell using ATP hydrolysis. ABCG2 also affects the pharmacokinetics of many drugs and contributes to multidrug resistance.[2]
Structural highlights
Overall Structure
ABCG2 is a homodimer with each monomer containing two domains, the nucleotide binding domain and the transmembrane domain , which are fused together as a single peptide chain.
[1] The NBD binds and processes ATP and is located inside of the cell where it is exposed to the cytosol. The TMD is responsible for binding and transporting any foreign substrates and is embedded in the cell membrane and extends into the extracellular region. (Figure 1).
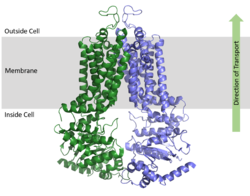
Figure 1. Orientation of ABCG2 in relation to the cell membrane.
(5NJ3) ATP Bound and Unbound Conformations
As an ABC Transporter, ABCG2 exhibits ATPase activity, using the energy of ATP hydrolysis to facilitate transport. After substrate bind in the TMD, one molecule of (2 molecules of ATP total) causing a conformational change of the overall structure from an to an . ATP coordinates with various residues and a magnesium ion in the . One molecule of ATP is hydrolyzed to transport substrates across the cell membrane while the second molecule of ATP is hydrolyzed to reset the transporter to its inward-facing conformation.[3]
When ATP binds, α-helices in the NBD approximately 35° relative to the . This shift in the NBD causes slight shifts of α-helices in the TMD; these helices are relative to the . The overall shift from inward-facing to outward-facing promotes the transport of substrates through the transporter.[2]
Cavities and Leucine Plug
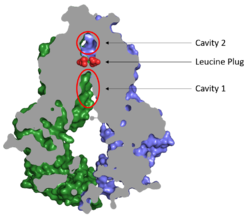
Figure 2. Locations of Cavities 1 and 2 and the Leucine Plug in ABCG2. The protein is in the inward-facing conformation with Cavity 1 open to the cytosol for substrate recruitment, the Leucine Plug is intact, and Cavity 2 is completely occluded.
(5NJ3)Substrates are transported through ABCG2 via two cavities separated by a leucine plug (Figure 2). acts as a multidrug binding pocket and is formed by helices at the interface of the monomers in the TMD. When ATP is not bound to the NBDs, Cavity 1 is in order to recruit substrates for transport. Cavity 1 is and full of nonpolar, hydrophobic residues and, as a result, prefers nonpolar, hydrophobic substrates, particularly flat, polycyclic molecules. Substrates, such as estrone sulfate, with residues from each subunit in Cavity 1.[1]
After substrates bind in Cavity 1, ATP binds each NBD leading to the transporter shifting from inward-facing to outward-facing. The outward-facing conformation results in the in the TMD in which the cavity is no longer . This collapse forces the substrate to move forward to Cavity 2 as there is no longer room in Cavity 1 to accommodate substrates.[2] , which is occluded when the protein in is the inward-facing conformation, is now open to the extracellular space and able to release the substrate. Cavity 2 contains a less hydrophobic environment and, as a result, substrates are released due to hydrophobic mismatch.[1] in the external loops near the exit of Cavity 2 also help promote substrate release.[2] Once Cavity 2 is empty, the protein reverts to the inward-facing conformation via hydrolysis of ATP.
Cavities 1 and 2 are separated by a which likely acts as a substrate check-point during transport; changes to either of these leucine residues have exhibited an increase in transport and a decrease in substrate specificity.[2] After the substrate binds Cavity 1 and ATP molecules bind each NBD, the to allow the substrate to enter Cavity 2. Once the substrate enters Cavity 2, the plug is able to reform and promote substrate release and conversion to the inward-facing conformation.
Disease
Dysfunctions in ABCG2 are linked to hyperuricemia which can lead to gout, kidney disease, and hypertension, all of which are thought to be the result of impaired transport of uric acid. Additionally, the expression of ABCG2 has been found to correlate with a poor prognosis and treatment outcome of various cancers including breast, ovarian, and lung.[4]
Cancer
ABCG2 hinders cancer treatment by contributing to multidrug resistance in tumor cells. ABCG2 exports xenbiotics, including vital anti-cancer drugs, which results in the inability to treat cancer cells. Cancer patients typically show high levels of expression of multiple ABC transporters. For example, acute myeloid leukemia (AML) has an increased expression of ABCB1, ABCG1, and ABCG2 while childhood AML shows an increased expression in ABCA3, ABCB1, ABCC3, and ABCG2.[5][6] Additionally, pancreatic cancer has shown an upregulation of ABCB4, ABCB11, ABCC1, ABCC3, ABCC5, ABCC10, and ABCG2.[7]
The substrate specificity among ABC transporters varies so this protein family can collectively export a wide variety of substrates and, ultimately, a wide variety of anticancer drugs. ABCG2 has been known to export anticancer drugs such methotrexate, mitoxantrone, topotecan, irinotecan, and flavopiridol[8]. Due to the high expression of multiple ABC transporters in cancer cells, simultaneous treatment of multiple transporters would likely be necessary for successful cancer treatment.
Inhibitors
ABCG2 hinders cancer treatment by contributing to multidrug resistance in tumor cells. ABCG2 exports xenbiotics, including vital anti-cancer drugs, which results in the inability to treat cancer cells. The inhibition of ABCG2 would stop the transport of anti-cancer drugs out of cancer cells. Due to the potential for ABCG2 inhibition to aid in cancer treatment, efforts have been made to develop specific inhibitors of ABCG2 and other ABC transporters. Some promising inhibitors were derived from fungal toxin fumitremorgin C; however, many of those derivatives developed have neurotoxic effects.[4]
ABCG2 that bind Cavity 1, acting as competitive inhibitors against ABCG2 substrates. Depending on the size of the inhibitor, one or two molecules can accommodate binding to the cavity and form within the binding site.[4] Many inhibitors are too big to be transported via the leucine plug resulting in the "clogging" of the transporter. With inhibitors acting as wedges, ABCG2 is locked in the inward-facing conformation and unable to transport molecules out of the cell.[2]


