Overview
The PWWP domain belongs to the Royal family, which consists of Tudor, chromodomain, MBT(Malignant Brain Tumor), and PWWP domains. The PWWP domain was first identified as a structural motif includes 100 to 130 amino acids in WHSC1 protein research. The researchers named after its very conservative sequence, Pro-Trp-Trp-Pro located at the β2 strand of protein.[1] This domain is mainly related to the recognition of methylated histone tail lysine and related to the epigenetic regulation of genes. Bromodomain and PHD finger-containing protein family is the group of proteins found in higher eukaryotes. The members have similar structural features and believed to be a essential part of interaction on H3K36me3.[2] Unlike BRPF1 and 2, BRPF3 is less studied by the researchers. Therefore many of its function remains unknown.
Structure
The main characteristic of PWWP domains is the two distinctive substructural motifs: β-barrel at the N-terminal region and helixes at C-terminal region.[3] The β-barrel is a very conservative feature of PWWP domains and the one at 3pfs is composed of 5 β-strands. The helixes at the C-terminal region are very different and 3pfs has 3 helixes which are little more than many of its relatives. The Pro-Trp-Trp-Pro motif, which is the most conservative, is little different in 3pfs, just like its closely-related proteins. The PWWP domains in BRPF family are composed of Tyr-Pro-Ser-Tyr and may suggest they would have a similar affinity. The stability of domain comes from both inter-substructure and intra-substructure interactions including hydrogen bonds and polar interactions. One unique feature of PWWP motif is that the first position affects the stability and aggregation of the protein. The proline gives more stability and oligomerization to the protein, compared to the alanine at the same position.[4]
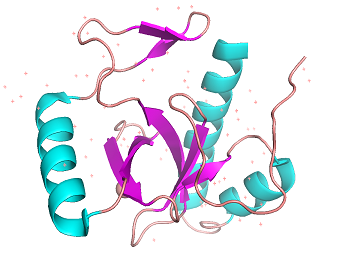
Figure 1A: Cartoon model of 3PFS motif. β-sheets(magenta) and helixes(orange) are shown. generated in PyMOL using PDB:
3PFS 
Figure 1B: The alignment of BRPF1 PWWP domain and BRPF3 PWWP domain. The green box indicates the 'PWWP' motif. generated in Tcoffee using PDB:
3PFS', '2X35 Function
Methyl-lysine reader
PWWP domains are one of epigenetic regulators which recognize specific lysine residues which are methylated. Even though there is no detailed research on 3pfs, it may be assumed to have similar function with BRPF1 PWWP domain which is shown to have methylated histone binding activity.[5] With a high-throughput mass spectrometry screening, this motif is suggested as an essential motif of histone 3 lysine 36 trimethylation binding.[6] The β-strands and Pro-Trp-Trp-Pro motif form hydrophobic cavity, which is the binding site of methylated lysines.[7] Since the aromatic cage is a common structure in the Royal family, it can be a common tool for methylated lysine binding.[8]
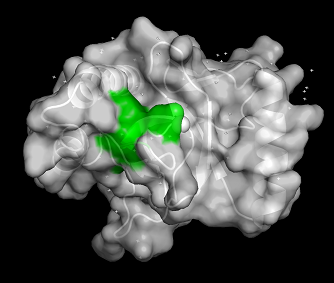
Figure 2: Surface of 3PFS with PWWP motif(green) is indicated. generated in PyMOL using PDB:
3PFS Nonspecific binding
The PWWP domains have a significant amount of basic residues which gives ability of nonspecific binding on DNA strands.[9] BRPF3 3pfs protein has 11 lysine residues and 12 arginine residues. These residues' sidechains remain being positively charged on in vivo pH. The DNA strand phosphate backbone, which is negatively charged, would be attracted to PWWP domain surface due to the nature of attraction between the opposite charges.
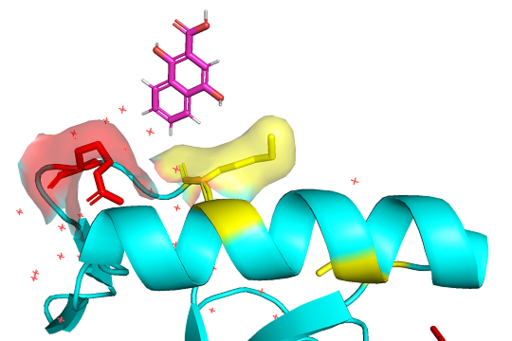
Figure 3: Lysine(yellow) and Arginine(red) residues on 3PFS surface interacting with DNA backbone generated in PyMOL using PDB:
3PFS
Evolutionary conservations
PWWP domain is only found in eukaryotes, from a yeast to a human. The number of PWWP containing protein is also varying on species; the human has 20. The loyal family has a common structural characteristic, three conserved β-strands.[10] The PWWP domain is also believed to be evolved from a common ancestor which had 3 β-strands. Using domain-tree approach, the BRPF family was branched out from rest of PWWP domain containg proteins and evolved itself.[11] As a domain, the PWWP presents in both unicellular and multicellular organisms. However, proteins of unicellular organisms are not found in multicellular creatures. Therefore, the PWWP domain was transmitted as a conserved linear arrangement alone, not the whole protein.[12]
Therapheutic features
There is no research on the relationship between the BRPF3 PWWP domain and any human disease. However, both of its closely related domains BRPF1 and BRPF2 PWWP domains are associated with hox gene regulation. BRPF1 PWWP domain presents on actively transcribing cells and may regulate Hox A9 gene which is related to MOZ-TIF2-dependant leukemia.[13] BRPF2 is also related to Hox gene wich is associated with schizophrenia and bipolar affective disorder.[14] The BRPF3 PWWP domain may also have Hox gene-related function since it has very similar β-barrel structure with its family members. However, a further research would be required for more understandings.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.




