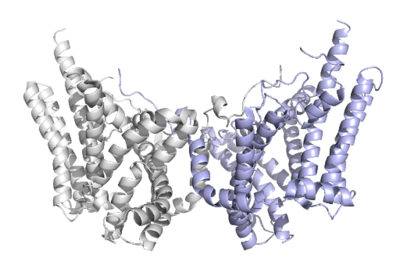
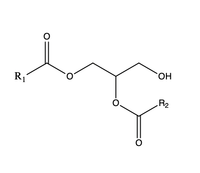
Diacylglycerol acyltransferase (DGAT) catalyzes the final and only committed step of triacylglycerol synthesis It does this by using diacylglycerol (DAG) and oleoyl CoA as substrates. DGAT plays a pivotal role in the metabolism of DAG and is important to the process of triacylglycerol metabolism. This metabolism is involved in intestinal fat absorption, lipoprotein assembly, lactation, and adipose tissue formation
Function
DGAT1 is a membrane protein responsible for the conversion of diacylglycerols to triacylglycerols.
Disease
Relevance
Structural highlights
Active Site
Tunnel System
There are two main tunnels that allow the enzymatic activity of DGAT to occur. The first is a cytosolic tunnel where the hydrophilic region of the oleyol-CoA binds to the cytosolic face that forms between helices TM6 and TM7. The CoA region sits at the cytosolic face with the fatty acid chain extending the rest of the way through the enzyme tunnel. When hydrophobic residues were substituted with the standard residues in the cytosolic tunnel, DGAT was completely inactivated.
The second is a that is orthogonal to the cytosolic tunnel. This tunnel is in the transmembrane region of the enzyme which allows lipids in the membrane to easily access the location. When a molecule of diacylglycerol (DAG), or another acyl acceptor, binds into this hydrophobic tunnel, the catalytic the transfer of the acyl group on the bound oleoyl-CoA to the DAG to form a triglyceride.
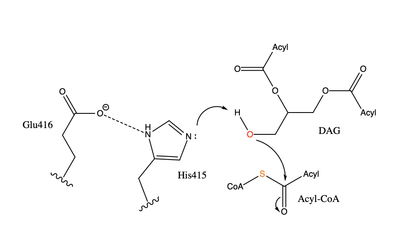
Mechanism
The catalytic deprotonates the hydroxyl group on the C3 of the glycerol backbone. The deprotonated oxygen then makes a nucleophilic attack on the carbonyl carbon of the Acyl-CoA, the electron density gets shifted up to the oxygen and back down to the carbonyl carbon. The sulfur of the Acyl-CoA takes the added electron density and the bond between the sulfur and carbonyl carbon is broken. Glu416 likely provides of the His415 despite being outside of the 3 angstrom hydrogen binding distance. This is likely due to the fact that the DGAT 1 enzyme was unable to be visualized with the diacylglycerol in the active site. The entrance and binding of the diacylglycerol may cause conformational changes and shifting of the Glu416 to become closer to the His415. Point mutations made to His415 support the hypothesis that it is essential for stabilization in the active site since the enzyme function was completely eliminated when the mutation was made.
Transition State Stabilization
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.