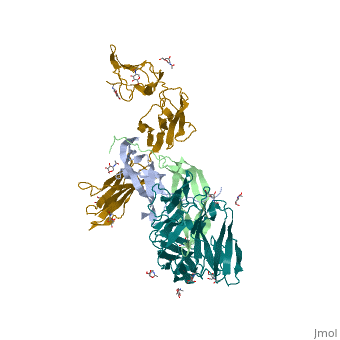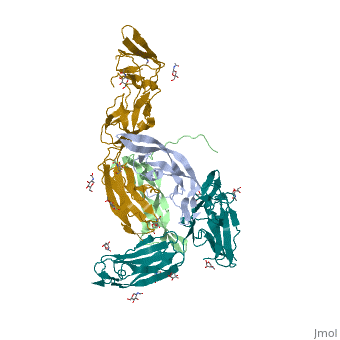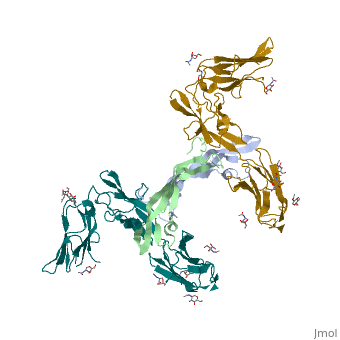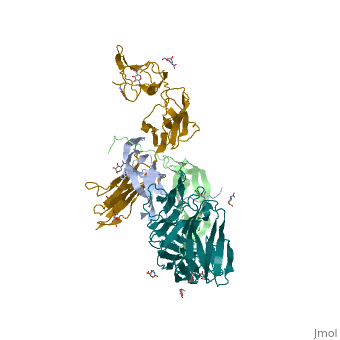
3D structure of the kinase domain of Activin receptor1 (Acvr1) complex with inhibitor shows with the protein including a and a [1]. Water molecule is shown as red sphere.
Rabbit [2]. Water molecules are shown as red spheres.
The structure of the complex between M-CSF and its receptor shows that a . There are [3]. .
The via the conserved kinase DFG motif (colored in salmon) and its gatekeeper threonine residue (colored in magenta)[4].
Lapatinib is a EGFR inhibitor used in breast cancer treatment. EGFRs are overexpressed in many types of human carcinomas including lung, pancreatic, and breast cancer, and are often mutated. This overexpression leads to excessive activation of the anti-apoptotic Ras signaling cascade, resulting in uncontrolled DNA synthesis and cell proliferation. The is responsible for activating this Ras signaling cascade. Upon binding ligands like Epidermal Growth Factor, EGFR dimerizes and autophosphorylates several tyrosine residues at its C-terminal domain. Upon phosphorylation, EGFR undergoes a significant conformational shift, revealing an additional binding site capable of binding and activating downstream signaling proteins.
Gefitinib inhibits the EGFR by located within the kinase domain. Residues Lys745, Leu788, Ala743, Thr790, Gln791, Met193, Pro794, Gly796, Asp800, Ser719, Glu762, & Met766 tightly bind the inhibitor. Unable to bind ATP, EGFR is incapable of autophosphorylating its C-terminal tyrosines, and the uncontrolled cell-proliferation signal is terminated.
Erlotinib inhibits the EGFR by located within the kinase domain. EGFR uses residues Asp831, Lys721, Thr766, Leu820, Gly772, Phe771, Leu694, Pro770, Met769, Leu768, Gln767 & Ala719 to tightly bind the inhibitor. Unable to bind ATP, EGFR is incapable of autophosphorylating its C-terminal tyrosines, and the uncontrolled cell-proliferation signal is terminated.
See also Herceptin - Mechanism of Action
Ephrins (Eph) are the membrane-bound ligands of ephrin receptors. The binding of Eph and ephrin receptors is achieved via cell-cell interaction. Eph/Eph receptor signaling regulates embryonic development, guidance of axon growth, long-term potentiation, angiogenesis and stem-cell differentiation [5]. Eph-A5 is implicated in spinal cord injury. Eph-A1 is implicated in myocardial injury and renal reperfusion injury. (PDB code 3mx0).[6]
. Water molecules are shown as red spheres.
.
(3fxx).[7]
Ephrin Type-A Receptor
The includes the N-terminal ephrin (Ligand)-binding domain (LBD), a cysteine-rich domain (CRD), and 2 fibronectin Type-III Repeats (FN3). EphA binds ephrins with . Most ephrins have a similar rigid structure which , AB, CD, FG, & GH. The LBD of EphA4 is said to be a “structural chameleon” able bind both A and B class ephrins. This explains why Ephrin Type-A receptors exhibit cross-class reactivity. The includes four important loops, the BC, DE, GH, & JK loops. EphA4 binds the GH loop of the ephrin ligand created by the EphA4 DE and JK loops. It is these loops, DE and JK, which undergo the greatest conformational shifts when binding either EphrinA2 or EphrinB2. , EphA4-Arg 162 forms a hydrogen bond with EphrinA2-Leu 138, while EphA4-Met 164 and EphA4-Leu 166 participate in hydrophobic interactions with EphrinA2-Leu 138 and EphrinA2-PHe 136. Although in the same binding pocket, the local interactions are significantly different. Most notably, the α-helix present in the EphA4-EphrinA2 JK loop is disrupted in the EphA4-EphrinB2 structure. This is due to that would occur between EphrinB2-Trp 122 and EphA4 Met 164. Instead, EphA4-Arg 162 and EphrinB2-Trp 122 form hydrophobic stacking interactions which stabilize the receptor-ligand complex. A morph of the movements EphA4 undergoes to bind EphrinA2 and EphrinB2 can be .
Eph-Ephrin complexes form two unique heterotetrameric assemblies consisting of distinct EphA2-EphA2 interfaces. is generated by . The 2nd involves complex and in the region .[8] These two heterotetramers generate a (). The proximity of kinase domains in an eph-ephrin tetramer, favors transphosphorylation of tyrosines in the cytoplasmic domains. Phosphorylation promotes kinase activity by orienting the activation segment of the kinase domain in a way that favors subsrate binding and subsequent signaling.
EPO is a glycoprotein composed of only . The sulfur of the cysteine residues links to form disulfide bonds. These disulfide bonds help keep EPO's structure. Helix A is connected to Helix D by , while Helix A and Helix B are connected by . EPO’s structure was determined in 1993. It is made up of four alpha helixes. EPO is produced mainly in the kidney, but further research has shown the brain and liver still produce small amounts.
See also: