Structure & Function
Domains
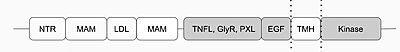
Fig.1 Anaplastic Lymphoma Kinase and its domains. The region from NTR to the MAM is the Heparin Binding Domain. The TNFL-PXL are the extracellular domains and the EGF is the domain that binds the extracellular region with the extracellular region of the transmembrane. The TMH is the transmembrane domain. The kinase domain is the intracellular portion of the ALK.
The extracellular portion of ALK has an inactive state, which is its monomerized form, and an active dimerized state with its ligands bound. The monomer is shown to the right in Figure 1, which has many different domains. The growth factor-like domain (EGF) connects the extracellular domains to the transmembrane domain (cyan). The tumor necrosis factor-like domain (TNFL) has a beta-sandwich structure that provides important residues that act as the binding surface for the ligand (orange). The glycine-rich domain (GlyR) contains 14 rare polyglycine helices that are hydrogen-bound to each other (green). The of these rare helices create a very rigid structure that is important for ALK function. The polyglycine extension loop (PXL) connects two of these polyglycine helices (pink).
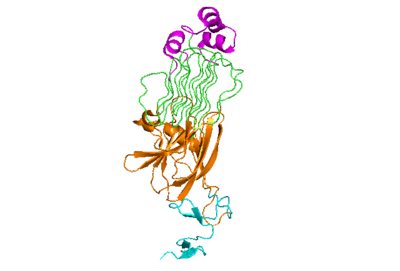
Figure 1: The ALK monomer unbound to ALKAL. Cyan: growth factor-like domain (EGF). Orange: tumor necrosis factor-like domain (TNFL). Green: glycine-rich domain (GlyR). Pink: polyglycine extension loop (PXL).
The domains that aren't shown in Figure 1 but are shown in the domain map (Figure 2) that also make up the monomer are the heparin binding domains (HBDs), which are at the N-terminal end of the monomer. Heparin has been found to be a possible activating ligand of ALK.[1] The transmembrane domain (TMH) are the residues of ALK that are located within the membrane. The kinase domain is the intracellular portion of ALK that contains the Tyr residues which are auto-phosphorylated when ALK is activated, initiating a signaling cascade.
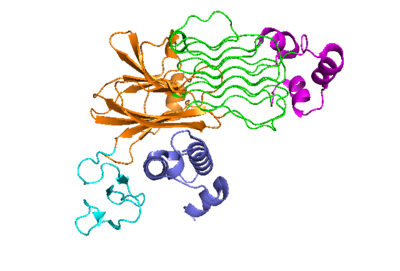
Figure 2: ALK-ALKAL complex, showing the conformation change of ALK from the binding of ALKAL.
Conformational Change
The anaplastic lymphoma kinase activating ligand (ALKAL) binds to the on the ALK at the TNFL domain. This induces a conformational change which allows for the PXL and the GlyR domains to hinge forward.[2] (Figure 2) ALK's TNFL has E978, E974, E859, and Y966 that form salt bridges with R123, R133, R136, R140, and R117 on ALKAL that allow for activation, leading to of two ALK-ALKAL monomers.
Membrane Guidance of ALKAL to ALK
The negatively charged phosphate groups on the cell membrane interact with a highly conserved positively charged on ALKAL that faces the membrane. These guides ALKAL to ALK and correctly positions ALKAL for its binding surface to face ALK's binding surface, which allows for a more favorable interaction. [3]
Role of Activated ALK
Once the ALKAL binds with ALK and dimerizes with another ALK-ALKAL complex, this activated conformation also initiates a conformational change of the intracellular kinase domain of ALK. This causes an autophosphorylation of several tyrosine residues of this domain, activating a signaling cascade with its kinase activity.
Disease
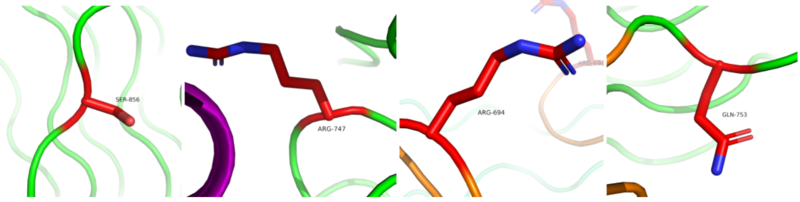
There are many that could take place causing constitutive receptor activation, enhancement between the interaction of receptors or stabilization of active receptors are known to relate to oncogenic potentials (Figure 4). The that is mutated to an arginine is known to be a gain-of-function in lung adenocarcinoma which can lead to constitutive activation of ALK. The that is mutated to the a serine may cause a gain-of-function mutation that is linked to acute myeloid leukemia. When the is mutated to a glutamate it is commonly identified in histiocytic neoplasms. The changing to arginine could cause possible oncogenic potentials which are not specified yet. The F856S and R753Q mutations are known to increase cytokine-dependent cell proliferation in certain cells. [4] [5]