Background
Ligands
Significance
Structure
Heterotrimeric G-Protein Structure
Novel Characteristics
MRGPRX2 demonstrates novel characteristics compared to other class A GPCRs. These structural motif differences contribute to a surface ligand binding rather than a ligand binding deep within the helices. To demonstrate this difference in depth binding, MRGPRX2 is compared to 5-HT2AR, another class A GPCR with more conserved structural motifs.
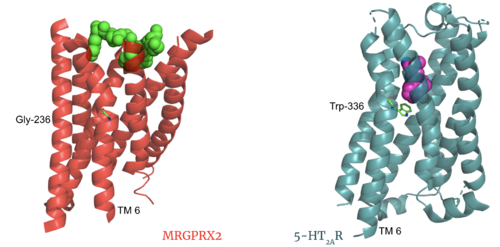
Comparison of ligand Cortistatin-14 binding in MRGPRX2 (left) and binding in 5HT2AR (right)
Toggle Switch
PIF/LLF Motif
DRY/ ERC Motif
MRGPRX2 has an rather than the typically conserved E/DRY Motif. The amino acid residue shift from TYR-174 to CYS-128 has spatial arrangement implications where the helices are more compact in MRGPRX2 without the TYR to physically push the TMP helices apart.
Sodium Site
The MRGPRX2 consists of ASP-75 and GLY-116 compared to the previously conserved residues in this binding pocket. Other class A GPCRs demonstrate a larger binding pocket with a higher negative character allowing for a suitable environment for sodium ions to bind. In MRGPRX2, this pocket lacks the same amount of with the shift to a glycine residue rather than typical negative residues. The helices for the MRGPRX2 in the binding pocket are also more collapsed making this pocket less accessible for sodium ions.
Disulfide Bonds
The MRGPRX2 disulfide bond is between CYS-168 and CYS-180 on TM helices 5 and 4, respectively. In other class A GPCRs, this disulfide bond is between and extracellular loop (ECL2) and a TM helix (TM3). For example, the shows this disulfide bond between the ECL2 and TM3. This different disulfide bond location contributes to surface level binding of ligands.

Overlay of the 5HT2AR and MRGPRX2 TMP for comparison of disulfide bond location.
Further Information
The MRGPRX2 GPCR also contains semi-conserved or fully-conserved motifs that are seen in other class A GPCRs.
NPxxY Motif
The in the NPxxY motif are pivotal for receptor activation in all Class A GPCRs. This motif is conserved in the MRGPRX2 receptor with residues VAL-231, ASP-75, ASN-275, and TYR-279.
CWxP Motif
The is almost fully conserved except for TRP-236, or the toggle switch, which is replaced with GLY-236. CYS-235, LEU-237, and PRO-238 are all conserved.
Function
GPCRs undergo a transmembrane protein conformational change upon ligand binding. This signal is then transduced to the G-protein allowing for downstream responses.
Before Activation
The culmination of different motifs observed in MRGPRX2 compared to other class A GPCRs leads to external membrane ligand binding.The MRGPRX2 GPCR undergoes a much smaller conformational change upon ligand binding compared to other Class A GPCRs due to surface level binding versus deep helix binding. This contrast is represented in the photo below to show the unbound and bound transmembrane proteins of MRGPRX2 and 5-HT2A.
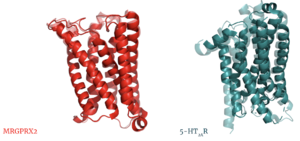
Overlay of unbound (transparent) and bound (opaque) transmembrane proteins of both MRGRPX2 (left) and 5-HT2AR (right).
After Activation
After ligand binding and transmembrane protein activation, this signal is sent to the alpha subunit of the G-protein which undergoes its own chemical and conformational changes. The alpha subunit is initially bound with GDP which then is physically replaced by GTP leading to conformational changes. These changes can be seen in the video below of a G-protein alpha subunit derived from common rats.
Clinical Relevance
3D Structures
References




