G-Protein Coupled Receptors
G-protein coupled receptors](GCPRs) are a large family of cell surface membrane proteins. Once bound to a wide variety of extracellular ligands, GCPRs undergo a conformational change and relay information to intracellular secondary messengers [1]. This G protein activation results in a cellular response dependent on the ligand bound and location of the GPCR in the body. GCPRs can be broken down into five families: the rhodopsin family (class A, 701 members), the secretin family (class B, 15 members), the adhesion family (24 members), the glutamate family (class C, 15 members), and the frizzled/taste family (class F, 24 members) [2]. All of the families have a similar transmembrane (TM) domain consisting of seven complexed with intracellular G proteins.
Class A GCPRs
Class A GPCRS or rhodopsin-like GPCRS are the largest and most studied type of GPCRS. Due to the diversity of these receptors they are found in many aspects of physiology. They are characterized by conserved motifs including DRY, PIF, Sodium Binding, and the CWxP [3]. A common example of a class A GPCRS is the β2AR receptor (PDB: 3sn6).
MRGPRs
The human itch GPCR, or Mas-related G-protein coupled receptor (MRGPR), is a Class A GPCR found in human sensory neurons and is responsible for the sensation of “itching” caused by skin irritation and diseases, insect bites, and hypersensitivity to certain drugs. MRGPR's are broken into 4 groups consisting of MRGPRX1, MRGPRX2, MRGPRX3, and MRGPRX4. MRGPRX4 is responsible for cholestatic itching, an intense itching felt during pregnancy on the soles of the feet and palms of hands. Meanwhile, MRGPRX2 regulates degranulation and hypersensitivity itch reactions [4]. These two, chiefly MRGPRX2, are often targets for drugs that result in mast cell degranulation and hypersensitivity side effects. In comparison to other Class A GPCRs, MRGPRX2 binds to an even wider range of ligands, including agonists such as cations and peptides.
Agonists
Cation
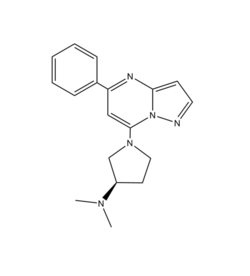
Figure 1: Structure of R-ZINC 3573. It is a cationic ligand that binds to receptor MRGPRX2.
[5] is a cation ligand that binds to MRGPRX2. Its N-dimethyl is inserted into a cavity of aromatic amino acid residues Phe-170, Trp-243, and Phe-244. In this , it makes ion pairs with Asp-184 and Glu-164. It is then stabilized by stacking its ring with Trp-248 and the Cys-168 to Cys-180 disulfide bond
[5].
Peptide
is one of the peptide ligands that binds to MRGPRX2. Cortistatin-14 interacts with the binding pocket through an interaction in sub-pocket 1 between Lys-3 on the peptide and Glu-164 and Asp-184 on MRGPRX2
[4]. Additionally, there are hydrophobic interactions in sub-pocket 2 between the peptide and the binding pocket due to the large hydrophobic amino acids on Cortistatin-14.
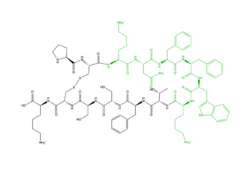
Figure 2: Structure of Cortistatin-14 with resolved amino acids highlighted in green.
[5] 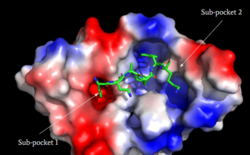
Figure 3: Binding pocket of MRGPRX2 with Cortistatin. There are two different binding pockets and cortistatin interacts with both of these through electrostatic interactions.
[4] Specific Traits
Disulfide bonds
In common Class A GPCRs the disulfide bond associated with the initiation of signal transduction is located on the extracellular domain of the 7 transmembrane helices. The of β2AR, a well studied Class A GPCR, occurs between (TM3) C106 and (EL) C191. This loop crosses through the middle of the extracellular domain, creating a barrier for bulkier substrates. In MRGPRX2, the is located between (TM4) C168 and (TM5) C180. This is a TM to TM disulfide bond as compared to a TM to EL disulfide bond seen in typical Class A GPCRs. This lack of interaction with the extracellular loop seen in MRGPRX2 causes the extracellular loop to flip on top of the TM4 and TM5 resulting in an open space for larger substrates to be able to interact with the receptor.
Toggle Switch
In β2AR, and other Class A GPCRs, there is a what is known as a “toggle switch” which puts a limit on how close the TM helices can get to each other due to tryptophan being a very large, bulky amino acid. This results in a deep binding pocket. In contrast, in MRGPRX2 Trp-286 is replaced by [4] [5]. Glycine is a much smaller amino acid and thus allows the helices to close the base of the binding pocket. This causes MRGPRX2 to have a very shallow binding site and consequently allows an even greater number of ligands to be able to bind. This can be seen in a shorter distance from the shorter distance from the ligand in β2AR compared to MRGPRX2.
PIF Motif
Another motif that MRGPRX2 differs from typical Class A GPCRs is the PIF/connector motif, which acts as a microswitch. PIF plays a role in connecting the binding pocket to conformational rearrangements required for receptor activation[6]. This motif is located towards the base of the TM domains. In a Class A GPCR, like β2AR, the consists of Phe-211, Ile-121, and Phe-282 on TM domains 5, 3, and 6, respectively. However, for MRGPRX2, this is replaced with Leu-194 on TM5, Leu-117 on TM3, and Phe-232 on TM6.
DRY Motif
The DRY motif is a proton microswitch that is located near the G-protein binding site C-terminal on TM3 [6]. It acts as an ion lock when the GPCR is not being activated, preventing unnecessary activation of the G-proteins [3]. This motif is conserved in typical Class A GPCRS however, in MRGPRX2 it is only partially conserved. The arginine is conserved, while the aspartate is replaced by a glutamate and the tyrosine is replaced by a cysteine [5] [7].
Sodium Binding
The sodium site motif is known to facilitate the conformational change of GPCR upon activation [8]. A sodium molecule sits in the middle of the TM7 helices where it is stabilized by conserved residues aspartate and glycine and 3 water molecules. In MRGPRX2 this motif is only partially conserved. The aspartate (TM2) is conserved while the glycine is replaced by a serine [5].
MRGPRX2 Signaling Pathway
MRGPRX2 mediates degranulation of mast cells through interaction with Gq and Gi subunits. When the subunit Gq is activated it activates Phospholipase C, which then catalyzes the cleavage of Phosphatidylinositol bi phosphate into diacylglycerol (DAG) and inositol trisphosphate (IP3). DAG is then able to increase the activity of protein kinase C. IP3 receptor is ligand gated calcium channel on the endoplasmic reticulum (ER), that causes the release of calcium in cytoplasm. Subsequently, this results in muscle contraction and enzyme activation.
MRGPRX2 also interacts with Gi or G-protein inhibitory, which is structurally homologous to Gs (G-protein stimulatory), and will inhibit adenylyl cyclase and therefore lowers (cAMP). Gi is stimulated when somatostatin binds to receptor in pancreas.
Clinical Relevance
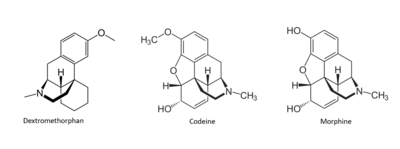
Figure 4: Structures of Dextromethorphan, Morphine, and Codeine.
[4] Many drugs have been linked to the activation of MRGPRX2 as a side effect that causes the sensation of itchiness. Among these drugs are morphine, codeine, and dextromethorphan.These drugs have a similar structure to that of (R)- ZINC-3573, introducing the idea of a similar binding mechanism. [9]
Due to mutations in key structural features of typical Class A GPCRS, MRGPRX2, it is able to bind to a variety of substrates that then mediate the signaling pathway for the sensation of itching.




