Sandbox reserved 1754
From Proteopedia
DNA RECOGNITION BY GAL4: STRUCTURE OF A PROTEIN/DNA COMPLEX
Structural highlights
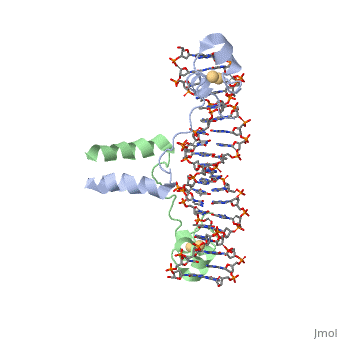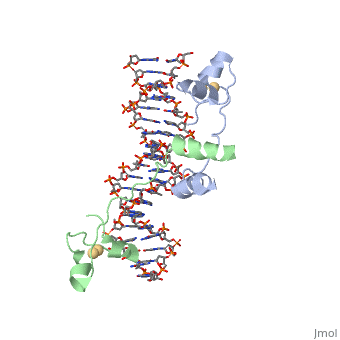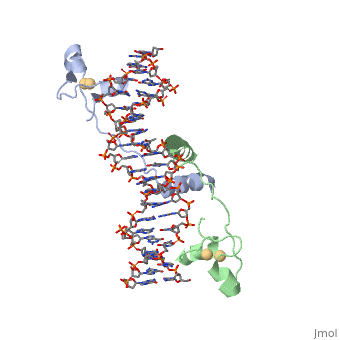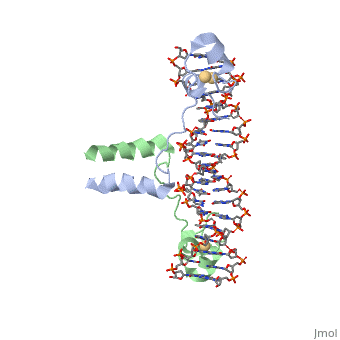
Function[GAL4_YEAST] This protein is a positive regulator for the gene expression of the galactose-induced genes such as GAL1, GAL2, GAL7, GAL10, and MEL1 which code for the enzymes used to convert galactose to glucose. It recognizes a 17 base pair sequence in (5'-CGGRNNRCYNYNCNCCG-3') the upstream activating sequence (UAS-G) of these genes. Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedA specific DNA complex of the 65-residue, N-terminal fragment of the yeast transcriptional activator, GAL4, has been analysed at 2.7 A resolution by X-ray crystallography. The protein binds as a to a symmetrical 17-base-pair sequence.Each subunit fold into three distinct modules: a compact, (residues 8-40), an (41-49), and an element (50-64). A small, Cd(2+)-containing domain recognizes a conserved CCG triplet at each end of the site through direct contacts with the major groove. The cadmium is coordinated to this domain via interactions with several . A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4. Gal4 also contains an upstream activating sequence () adjacent to that of the promoter region. This sequence works much like an enhancer regions that are common in Eukaryotic genes.The sequence of this UAS appears to be similar to previously determined UAS sequencs, but not quite identical. Some major motifs can be seen in the bases that are interacting with the DNA. Such as, bases 31-35 express a sequence of TCCTC. The protein also appears to not interact with both strands of DNA simultaneously, but rather depends on which half of the dimer is being looked at. DNA recognition by GAL4: structure of a protein-DNA complex.,Marmorstein R, Carey M, Ptashne M, Harrison SC Nature. 1992 Apr 2;356(6368):408-14. PMID:1557122[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
RecA Structure and Function
RecA is one of the many proteins that is involved in recombination cross over events and during recombination repair in response to single strand DNA breaks. RecA is a rather small monomer protein that can multiplex with itself up to thousands of RecA proteins in order to associate with either dsDNA or ssDNA. The structure of RecA was determined through x-ray crystallography and each monomer contains very distinct structural components. These components are a largely helical 30-residue N-terminal region, a 240-residue α/ß ATPase core, and a 64-residue C-terminal globular domain. The process of recruiting new RecA monomers is carried out an ATP dependent process. This occurs through the binding of ATP to two different α/ß ATPase cores on subsequent RecA monomers. "INSERT HERE HOW ATP IS COORDINATED". Once several RecA monomers have been attached to one another, they coordinate with ssDNA to form a a repeating structure that contains exactly three nucleotides for every RecA monomer. However, this does not mean that each nucleotide triplet only interacts with a single RecA monomer. In reality, each RecA monomer spans three nucleotides, but the nucleotide triplet interacts with the other two RecA surrounding it on both the 5' and 3' sides. Essentialy, each nucleotide triplet is interacting with three different RecA monomers. When ssDNA is bound to RecA in this manner it take a unique conformation becoming very similar to the normal B-DNA structure, however, the bound DNA is stretched slightly from normal parameters. RecA binds DNA through interactions with the phosphate backbone.
See AlsoReferences
| ||||||||||||||||||||||||