This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Background
Structural Overview
There are two significant areas in the NTCP structure that facilitate ligand binding, which are referred to as "patches." Residues 84-87 of NTCP are patch 1, which are located on the TM2-TM3 loop in the core domain. This is also considered the extracellular region of NTCP “tunnel." Residues 157-165 NTCP are associated with patch 2. They are located on N-terminal half of the TM5 in the panel domain (residue sequence: KGIVISLVL). Patch 2 is also located in th extracellular region. These residues' importance was determined through mutations of these residues and examined through pull-down assays (Asami, et. al, 2022).
Binding Pocket
Confirmation Change
Mechanism
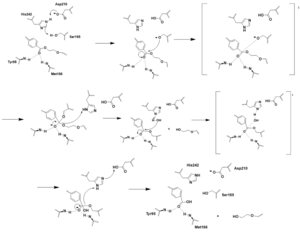
Figure 1. Bile Salt Uptake Mechanism.
The NTCP protein goes through a conformational change when assisting in the uptake of bile salt into the cell. This is accomplished through the opening of a wide transmembrane pore, creating a transport pathway for bile salts. The mechanism includes two sodium metal ions that allow for residue stabilization when going through the conformational change. Binding of the preS1 region of the HBV/HDV virus blocks any subsequent bile salt uptake. Thus, preS1 binding blocks the conformational change and entry of any salts into the cell. Residues 8-17 of preS1 are critical for NTCP:pres1 binding. Patch 1 and Patch 2 (external) residues interact with residues 8-17 of preS1 to facilitate binding.
Function
Significance
NTCP serves a multitude of biological functions, including bile salt uptake and HBV/HDV binding.
Bile Salt Uptake
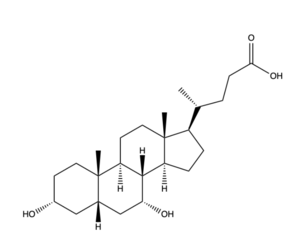
Figure 2. Bile Salt Structure.
HBV/HDV


