Introduction
Sodium Taurocholate Co-Transporting Polypeptide, or NTCP, is a membrane transporter protein that is found in the plasma membrane of liver cells, or hepatocytes. NTCP's primary function is the transportation of taurocholates, or bile salts, into the liver and out of the liver to the small intestine [1] NTCP is part of the solute carrier superfamily, more specifically SLC10. NTCP is the founding member of the SLC10 family, first discovered in rat hepatocytes in 1978 [2] NTCP has a key role in Enterohepatic circulation, and it's unique ability to transport other solutes lends it therapeutic potential for lowering cholesterol and liver disease.
NTCP also serves as a binding site for hepatitis B virus and hepatitis d virus [3] Future studies into HBV binding mechanism can help understand infection pathways and the development of viral inhibitors.
Structure
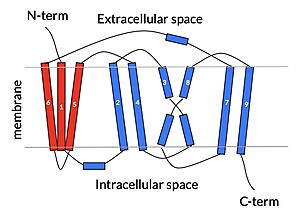
cartoon depiction of NTCP topology
Overview
Sodium Taurocholate Co-Transporting Polypeptide, or NTCP, is one continuous polypeptide chain consisting of a total of 9 transmembrane α helices (TM1-9). The N-terminus of the polypeptide chain is found on the extracellular side of the plasma membrane while the C-terminus is located on the intracellular side. There are two distinct domains within the quaternary structure of NTCP: a (blue) and a (red), both being a part of the same polypeptide chain. The core domain includes 6 transmembrane α helices (TM2-4 and TM7-9) and demonstrates two-fold pseudosymmetry while the panel domain consists of 3 transmembrane α helices (TM1 and TM5-6) and does not display symmetry. Within the core domain, there is a unique crossover between TM-3 and TM-8 that is known as the . This motif is important because this is where the transporter's substrate binding site is located which will be discussed in more detail.
Active Sites
NTCP, among others in the SLC10 family, have . Many polar and negatively charged residues are characteristic of these active sites. The high level of conservation among sodium binding placement and interacting residues suggests sodium binding is coupled to bile salt transport. Additional mutations in the X-motif near sodium binding sites have shown that bile salt transport function is lost also suggesting that sodium allows bile salt binding.
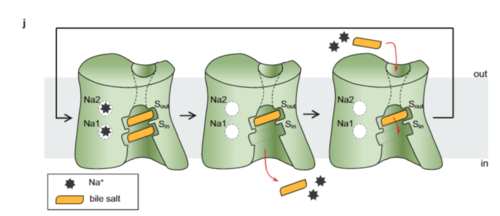
[4] It is understood that these sodium binding sites facilitate changes from open-pore to closed pore states of NTCP that allow for the binding or release of bile salts. Closed-pore state is favored in the absence of sodium ions, while open-pore state is favored in the presence of sodium ions. This also allows for sodium concentrations to regulate uptake of taurocholates. When intracellular sodium levels are higher, open-pore state is favored allowing for the diffusion of taurocholates. However, when extracellular sodium levels are high, closed-state is favored preventing diffusion of taurocholates. [4]
The is also characteristic of NTCP. The pore surface remains Hydrophobic, while lining of the open pore state is largely Polar. This pattern is believed to follow similar amphipathic patterns within taurocholate and other NTCP substrates, such as steroids and statins. [4] Thus the channel provides specificity while preventing leakage of other substrates. When observing the relevant it is shown that some residues form Van der waals interactions while others will form dipole-dipole or ionic interactions with bile salt substrates. The core domain appears to contribute most of the polar domains, while the panel domain contributes more hydrophobic residues.
Conformational Change
Bile Salt Transport
Medical Relevancy
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.