Kaushki Sharma- BI3323
From Proteopedia
Interactive 3D Complement in Proteopedia
|
| |
|
Cryo-EM structures of human OAT1 reveal drug binding and inhibition mechanisms[1]. | |
|
Cell Volume 33, Issue 11, P1856-1866.E5, November 06, 2025 |
Structure Tour
Classification: MEMBRANE PROTEIN
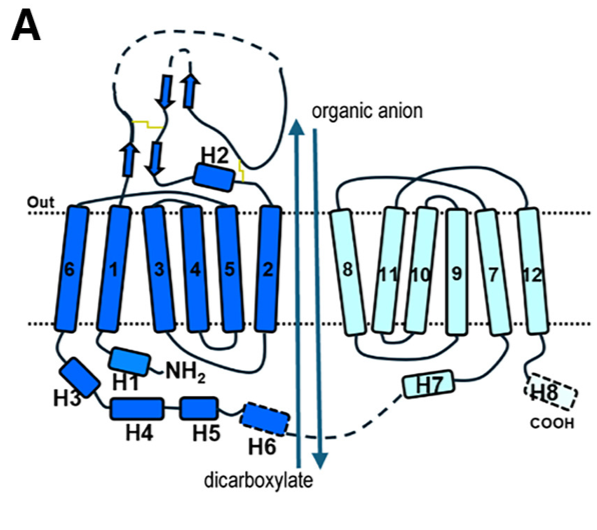
IntroductionMembers of the organic anion transporter (OAT) family, including OAT1, are expressed on the epithelial membrane of the kidney, liver, brain, intestine, and placenta.[2][3] OAT1 regulates the transport of organic anion drugs from the blood into kidney epithelial cells by utilizing the α-ketoglutarate (α-KG) gradient across the membrane established by the tricarboxylic acid (TCA) cycle.[4] [5]OAT1 also plays a key role in excreting waste from organic drug metabolism and contributes significantly to drug-drug interactions and drug disposition. However, the structural basis of specific substrate and inhibitor transport by human OAT1 (hOAT1) has remained elusive. Here are four cryo-electron microscopy (cryo-EM) structures of hOAT1 in its inward-facing conformation: the apo form, the substrate (olmesartan)-bound form with different anions, and the inhibitor (probenecid)-bound form. Organism(s): Homo sapiens Expression System: Homo sapiens Mutation(s): No Deposited: 2024-11-13 Released: 2025-11-05 Deposition Author(s): Jeon, H.M., Eun, J., Kim, Y. Funding Organization(s): National Research Foundation (NRF, Korea) Experimental Data Snapshot Method: ELECTRON MICROSCOPY Resolution: 3.85 Å Aggregation State: PARTICLE Reconstruction Method: SINGLE PARTICLE Cryo-EM structure of hOAT1The apo state structure of human Organic Anion Transporter 1 (hOAT1), determined by cryo-EM, reveals the transporter in an inward-facing conformation. This means the central substrate-binding cavity is open toward the intracellular side of the membrane, ready to release a substrate or accept one from the cytoplasm. Key Structural Characteristics:
Olmesartan recognition by hOAT1The structural and functional analysis of provides a detailed blueprint for substrate specificity and binding.
Mechanism of OAT1 inhibition by probenecidThe cryo-EM structure of reveals a dual-mechanism of action that goes beyond simple competition, effectively arresting the transporter in a restricted state. 1. Binding Mode and Direct Competition
2. Conformational Arrest and Cytoplasmic Path Blockage The primary inhibitory mechanism is a probenecid-induced conformational change that physically blocks substrate access and exit. Compared to the apo state, the cytoplasmic opening of the binding pocket narrows from ~15 Å to ~12 Å in the probenecid-bound state. Probenecid binding narrows Path A and completely blocks Path B. Restriction of the access route to path B likely limits the entry of substrates to Site 1 and the exit of substrates from the binding pocket. This structural rearrangement is caused by a slight inward movement of the cytoplasmic ends of TM5, TM8, TM10, and TM11 toward the binding pocket. 3. Locked Conformation By constricting the cytoplasmic access routes, probenecid does not just compete for the substrate-binding site; it stabilizes the transporter in an apo-like, inward-facing conformation that is inaccessible to cytosolic substrates. This prevents the entry of new substrates and likely traps the transporter in this non-functional state, effectively "locking" it and preventing the conformational changes necessary for the transport cycle. Mechanistic Insights into hOAT1 Function and Inhibition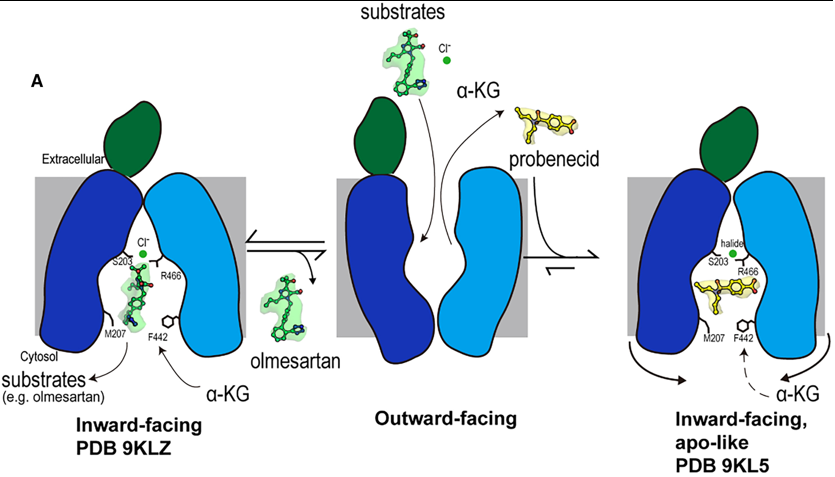 Fig 2. Mechanism of olmesartan binding and conformational inhibition by probenecid. A) When the transporter is in its outward-facing conformation, substrates or inhibitors enter the central binding pocket and undergo structural rearrangement to the inward-facing conformation. When olmesartan interacts with the bottom gating residues M207 and F442, the side chains S203, Y230 (not shown here), and R466 appear to rearrange to coordinate with a chloride ion and drug compared to the apo structure. Whereas probenecid binding induces an additional conformation change for inhibition (apo-like conformation). A Dual-Mechanism for Potent Inhibition by Probenecid The study reveals that the classic inhibitor probenecid employs a sophisticated, dual-mechanism to arrest OAT1 function, moving beyond simple competition. Direct Competition: Probenecid occupies the central binding pocket, and its interaction with K382 in Site 1 directly competes with the binding of the counter-substrate α-ketoglutarate (α-KG). This disrupts the exchange cycle that drives substrate transport. Conformational Arrest: More significantly, probenecid binding induces subtle conformational changes in the cytoplasmic ends of transmembrane helices (TM5, TM8, TM10, TM11). This leads to a constriction of the cytosolic opening, completely blocking one access path (Path B) and narrowing the other (Path A). This physically prevents substrates from entering or exiting the binding site from the cytoplasm, effectively "locking" the transporter in an inactive, inward-facing state. This mechanism is reminiscent of inhibition seen in other transporters like hURAT1, suggesting it may be a general strategy for effective transport arrest. ConclusionrOAT1 structures with probenecid have been reported previously, [6] and our hOAT1 structures align with findings for rOAT1 and provide new insights into the mechanism by which probenecid inhibits transport activity. Additionally, this study reveals the structure of hOAT1 with olmesartan, offering mechanistic insights into species-specific differences in OAT1 transport of specific substrates.
Notes & References
| ||||||||||||