Sandbox Aryan 20221057 BI3323-Aug2025
From Proteopedia
|
Contents |
Structure Overview
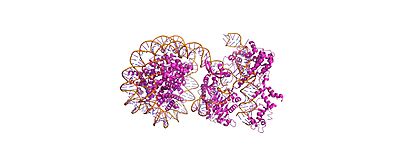
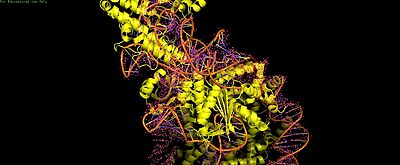
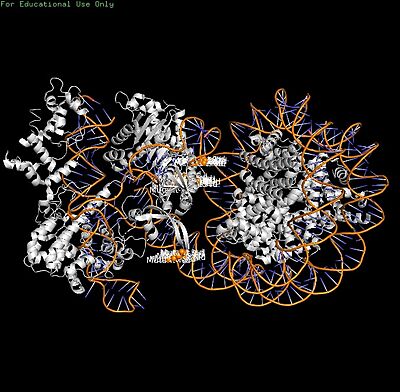
Cas9-sgRNA ribonucleoprotein targets nucleosome **linker DNA** (PAM1/PAM28) and entry-exit regions (SHL6), avoiding tightly wrapped **core DNA** (SHL0-5). Native-PAGE on Widom 601 nucleosomes confirmed preferential cleavage at DNA ends where transient unwrapping occurs.[attached_file:1]
The post-cleavage complex shows HNH/REC2 domains disordered, bridge helix absent, and target/non-target DNA strands cleaved—consistent with binary biochemical data.[web:2]
PI Domain Interactions
Cas9's PI domain (residues ~1100-1368) makes multiple contacts: - Histone tails: Weak electrostatic interaction (non-essential for binding) - PI edge (K1155): Lysine stabilizes post-cleavage complex via DNA phosphate backbone - Core DNA loops (H1264/R1298/K1300): Nonspecific binding inhibits cleavage
- Mutagenesis validation**: H1264A/R1298Q/K1300A mutants increase nucleosome binding AND cleavage efficiency both in vitro and rice callus genome editing.[attached_file:1]
Dual Inhibition Mechanism
1. Access barrier: Nucleosome DNA ends inflexible (SHL0-5), blocking Cas9 binding 2. Motion restriction: PI-core DNA trapping limits HNH/RuvC domain movements for cleavage
Entry/exit asymmetry from Widom601 sequence flexibility explains variable editing across chromatin contexts.[web:14]
Implications
Reveals Cas9's eukaryotic adaptation strategy and identifies **chromatin-optimized variants** for improved genome editing tools.[web:121]
BI3323-Aug2025