This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Factor IX
From Proteopedia
-----This page is still under construction-------
|
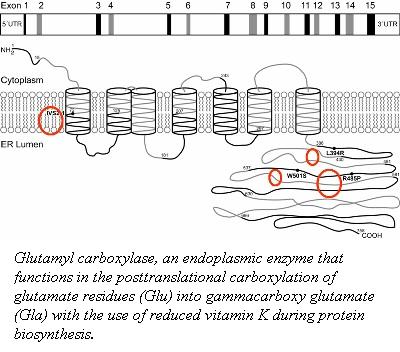
Factor IX (plasma thromboplastin component, Christmas factor, or hemophilia B factor) is a 57-kDa vitamin K-dependent procoagulant glycoprotein. It is synthesized by the liver hepatocyte as a pre-prozymogen that requires extensive posttranslational modification. The pre-prozymogen contains a pre-peptide (hydrophobic signal peptide) at its amino terminal that transports the growing polypeptide into the lumen of the Endoplasmic Reticulum. Once inside the ER, this signal peptide is cleaved by a signal peptidase. A pro-peptide functions as a recognition element for a vitamin K-dependent carboxylase (γ-glutamyl carboxylase) which modifies 12 glutamic acid residues to gamma-carboxyglutamyl ( ) residues. These residues are required for the association with the anionic phospholipid surface through Ca2+-dependent binding. The Domain is followed by two epidermal growth factor domains ( and ). The N-terminus of contains a Ca2+ binding site, while the C-terminus connects to a hydrophobic pocket of and a salt bridge through Lys122 ( residue) and Gln74 (). connects to the domain through a linker peptide and is required for a proper orientation and folding of . To have a physiologically active factor IX, two cleaves must occur to remove a 35 amino acid region that precedes the catalytic region. The first cleave is at Arg145, generating an inactive FIXα. The second cleavage is at Arg180 results in a catalytically active molecule FIXaβ. This resulting heterodimer is held by a disulfide bridge at Cys132-Cys289. The contains a catalytic triad of . Upon cleave at Arg180, Val181 can form a salt bridge with Asp364, which is a characteristic of active . The active FIXa, can then interact with its cofactor, FVIIIa, to form a membrane-bound Xase complex, which activated FX to FXa.
Gene Structure and Expression:
The gene for factor IX is located on the long arm of chromosome X between positions 26.3- and 27.1 and contains eight exons and seven introns, which segregate the FIX gene into specific structural regions.
| I | II | III | IV | V | VI | VII | VIII |
Exon I encodes the hydrophobic signal peptide that targets the FIX into the lumen of the Endoplasmic Reticulum. Exon II codes for the pro-peptide and the Gla domain. Exon III encodes the , that inserts itself into the lipid membrane, anchoring FIX. Exon IV and V, code for EGF-1 and EGF-2, the serine protease region is encoded by exon VI-VIII.
Carboxyglutamate: Post-translational carboxylation of glutamate residues (Glu) into gammacarboxy glutamate (Gla)
Cite error: Invalid <ref> tag;
invalid names, e.g. too many
Proteopedia Page Contributors and Editors (what is this?)
Nadia Dorochko, Michal Harel, Alexander Berchansky, David Canner