MDM2
From Proteopedia
MDM2 BOUND TO HUMAN P53 Protein
About MDM2 Structure
The MDM2 oncoprotein is a cellular inhibitor of the p53 tumor suppressor in that it can bind the transactivation domain of p53 and downregulate its ability to activate transcription. In certain cancers, MDM2 amplification is a common event and contributes to the inactivation of p53. The crystal structure of the 109-residue amino-terminal domain of MDM2 bound to 109-residue transactivation domain peptide of p53 revealed that MDM2 has a on which the p53 peptide binds as an amphipathic alpha helix. The interface relies on the steric complementarity between the MDM2 cleft and the hydrophobic face of the p53 alpha helix and, in particular, on a triad of p53 amino acids-Phe19, Trp23, and Leu26-which insert the MDM2 cleft. These same p53 residues are also involved in transactivation, supporting the hypothesis that MDM2 inactivates p53 by concealing its transactivation domain. The structure also suggests that the amphipathic alpha helix may be a common structural motif in the binding of a diverse family of transactivation factors to the TATA-binding protein-associated factors.
Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain., Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP, Science. 1996 Nov 8;274(5289):948-53. PMID:8875929
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
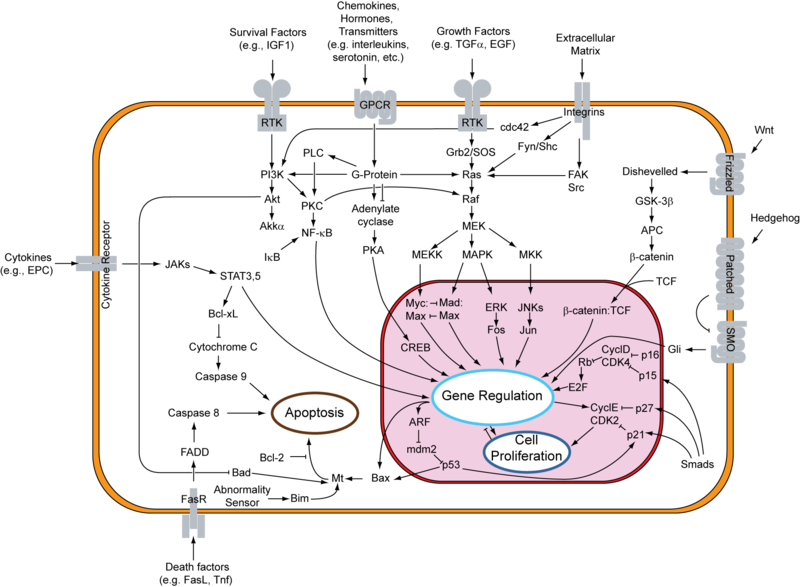
==Role in Cancer==MDM2 is an active inhibitor of the p53 anti-tumorgenesis protein. When MDM2 is atached p53 is unable to release transcription factors that code for apoptosis or halting of the cell cycle. The full cell signaling pathway is very complex, but MDM2 is usually activated by
Reference
- Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996 Nov 8;274(5289):948-53. PMID:8875929
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Matt Routh, David Canner, Joel L. Sussman, Yousuf Bahrami, Alexander Berchansky