Alendronate
From Proteopedia
| Please do NOT make changes to this Sandbox. Sandboxes 30-60 are reserved for use by Biochemistry 410 & 412 at Messiah College taught by Dr. Hannah Tims during Fall 2012 and Spring 2013. |
Contents |
Alendronate (Fosamax®)
Alendronate is commonly known for its use in treatment and prevention of osteoporosis in postmenopausal women and men, but is also used to treat Paget's disease (disease that results in deformed and enlarged bones).[1] Alendronate belongs to the class of nitrogen-containing bisphosphonates, which are inorganic pyrophosphate analogues.
History of Bisphosphonates
Bisphosphonates were first synthesized in Germany in 1865, but were not studied biologically until 1968. In the interim time, they were used in the textile and fertilizer industries due to their apparent inhibitory effect on calcium carbonate. However, in 1968, a group in Switzerland found inorganic pyrophosphates in urine and plasma. In vitro testing of these molecules revealed that they inhibited calcium phosphate precipitation and dissolution, but were destroyed in vivo by phosphatases. These results led to the discovery of bisphosphonates, as they reacted in the prescribed manner.[2]
Sodium alendronate was first marketed in 1994 as Fosamax® by Merck pharmaceutical. In 2008, Merck lost their U.S. patent on alendronate, allowing Barr Pharmaceuticals and Teva Pharmaceuticals USA to begin marketing generic forms of sodium alendronate. Other brand names for the drug include Fosamax+D®, Adronat, Alendros, Arendal, and Onclast.[3]
Structure
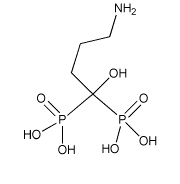 [4] Alendronate is a nitrogen-conatining bisphosphonate with
[4] Alendronate is a nitrogen-conatining bisphosphonate with
Target Protein
|
Natural Substrates
Isopentenyl pyrophosphate (IPP) actually binds to and stabilizes the alendronate-FPPS complex, rather than competing with the inhibitor.
Side affects of Drug
References
- ↑ http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0000018/
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC138713/?tool=pmcentrez
- ↑ http://www.drugbank.ca/drugs/DB00630
- ↑ Image from: http://pharmacy-and-drugs.com
- ↑ Image from: http://reference.findtarget.com

