User:Xuni Li/Sandbox 1
From Proteopedia
|
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground.
Contents |
Introduction
Bacteria use their receptors to sense the environment to change their swimming patterns. There are different kinds of chemoreceptors that respond to different stimuli. The figure on the right is the methyl-accepting protein of Thermotoga maritima receptor. Bacteria like to flee away from the repellent when high concentrations are present in the environment. CheA is a histidine kinase that associates with CheW, an adaptor protein, will cause the flagella to turn clockwise and result in a tumbling motion. On the other hand, when a high concentration of attractants are present in the environment, the CheA kinase will be turned off, cause flagella to turn counterclockwise, resulting in a forward swimming pattern. [1]
Structure and Functions
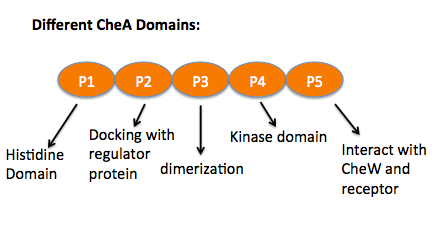
Chemoreceptors usually contain a periplasmic ligand binding domain, the transmembrane domain, a HAMP domain which is for signal conversion, and the cytoplasmic domain that contains the methylation sites, flexible bundle and protein binding sites where CheA and CheW bind. CheA is a histidine kinase that contains five units. The P1 domain is the site of substrate autophosphorylation that associates with kinase P4 domain, P2 is where phosphate transfers to CheY, another response regulator protein, from P1. P3, P4 and P5 are the dimerization, kinase and the receptor-coupling domains. The P3 domain was predicted to interact with CheW which stabilize the interface between P3 and P5. The NMR structure has shown that P5 is proximal to the CheW β barrel (residues 635-660). [2] On the right, is the CheW-CheA P4, P5 domain superimposed with CheW-CheA P3, P4, P5.
Metazoans adapt to oxygen levels in the environment by making use of intracellular oxygen levels as signals to regulate the transcription of genes that are essential under normoxic or hypoxic conditions. Central to this mechanism is the oxygen-dependent hydroxylation on specific proline and asparagine residues of the transcription factor, hypoxia-inducible factor (HIF)-α.[3]
Prolyl hydroxylase domain (PHD) enzyme (EC 1.14.11.-) is a Fe(II)/2-oxoglutarate (OG)-dependent dioxygenase that catalyzes the trans-4-hydroxylation of the specific proline residues (in humans, Pro-402 and Pro-564) in (HIF)-α. In addition to iron, this enzyme also requires ascorbate as a cofactor.[2]
PHDs belong to the same oxygenase superfamily as the collagen prolyl hydroxylases. Inside the cell, these proteins are mostly found in the cytoplasm in contrast to collagen prolyl hydroxylases, which reside in the endoplasmic reticulum. In mammals, the PHD dioxygenase subfamily originally includes three homolog members but was recently updated to include another member: PHD1 (also known as HPH3 and EGLN2), PHD2 (also known as HPH2 and EGLN1), PHD3 (also known as HPH1 and EGLN3), and a newly identified enzyme called P4H-TM (also recently named PHD4 and EGLN4). Both PHD1 and PHD2 contain more than 400 amino acid residues while PHD3 has less than 250. All isoforms, however, contain the highly conserved hydroxylase domain in the catalytic carboxy-terminal region. [3]
Molecular Playground banner: Prolyl Hydroxylase Domain (PHD) enzyme, a cellular oxygen sensor, has a major regulatory role in oxygen homeostasis.
Structure
PHDs have two structural domains: the more variable N-terminal domain and the conserved catalytic C-terminal domain. The catalytic domain core of PHDs consists of eight β-strands in a "jelly-roll" or double stranded β helix supported by three conserved α-helices and other β-strands and loops that pack along the core. Possession of the DSBH motif is typical of 2-OG-dependent oxygenases. Contained in this core are the three Fe(II)-binding ligands formed by the conserved triad sequence, His-X-Asp/Glu-Xn-His.[3][4][2]
The , which is located on a deep cleft between the β-strands comprising the DBSH core, contains the essential Fe(II). It is normally coordinated by the conserved two-histidine-one-carboxylate , 2-OG and a water molecule to form an octahedral geometry. Aside from the triad motif residues and those that bind 2-OG, the residues that are predominant inside the active site are nonpolar in nature. This is evidence of the enzyme's need to protect the protein core from oxidation by reactive species that are sometimes generated from iron-related reactions like the Fenton type reaction.[2]
Function
The intrinsic dependence of PHD-catalyzed hydroxylation reactions on molecular oxygen concentration led to the most notable role of PHDs as cellular oxygen sensors. The hydroxylation happens at position 4 of the residues Pro-402 and Pro-564 located in the C-terminal oxygen-dependent degradation domains (ODDs) of the hypoxia-inducible transcription factor, (HIF)-α.[3]
The requirement of PHDs for the TCA cycle intermediate, 2-oxoglutarate, also opens the possibility of these enzymes acting as regulators of processes that relate metabolic activity to oxygen levels. Aside from regulation of oxygen homeostasis, other biological functions of the enzyme, which may be hydroxylase-independent or still hydroxylase-dependent but (HIF)-α-independent, are being proposed. This is mainly based on the results of various studies: some showed that other factors such as nitric oxide, reactive oxygen species (ROS), and several oncogenes control PHD oxygenase activity[5]; while others described PHD activity on other substrates like IKK-β[3]. In fact, several functions of the enzyme have been recently identified based on these studies. Listed below are the currently identified functions for PHDs in general[3]:
- tumor suppressor
- promoter of cell death (apoptosis)
- regulator of cell differentiation
3D structures of prolyl hydroxylase domain
References
- ↑ Hazelbauer, Falke and Parkinson. "Bacterial chemoreceptors: high-performance signaling in networked arrays." Biochemical Sciences, 2007, 33 (1), 9-19. PMID:[1]
- ↑ 2.0 2.1 2.2 2.3 Park, Borbat, Gonzalez-Bonet, Bhatnagar, et al. "Reconstruction of the chemotaxis receptor-kinase assembly." Nature Structural and Molecular Biology, April 23, 2006, 13 (5), 400-407. PMID:[2]
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Fong, G.H., Takeda, K. "Role and Regulation of Prolyl Hydroxylase Domain Proteins." Cell Death and Differentiation, February 15, 2008, 15, 635-641. PMID:18259202
- ↑ Schofield, C.J., Ratcliffe, P.J. "Signalling Bypoxia by HIF Hydroxylases." Biochemical and Biophysical Research Communications, August 24, 2005, 338, 617-626. PMID:16139242
- ↑ Kaelin, W.G. "Proline Hydroxylation and Gene Expression." Annu.Rev.Biochem., February 8, 2005, 74, 115-128. PMID:15952883