Sandbox 212
From Proteopedia
Carnitine acyltransferases are a large family of enzymes that play a main role in cellular energy metabolism, i.e. fatty acid oxidation. These enzymes catalyze the reversible exchange of acyl groups between coenzyme A and carnitine. Carnitine acyltransferases include three different classes of enzymes which are known as carnitine acetyltransferases (CrATs), carnitine octanoyltransferases (CrOTs) and carnitine palmityltransferase (CPTs). The three classes differ in their acyl group specificity as well as their localization. [1] In this article we will focus on the structure of carnitine acetyltransferase as a representitive of carnitine acyltransferases. Determining the structure and thus the molecular basis for fatty acid transfer is needed for drug development.Being major enzymes in fatty acid oxidation carnitine acyltransferases are viewed as promising targets which can be used to develop successful therapeutics against type 2 diabetes, obesity and other human diseases.
Contents |
Biological function
- Differences within carnitine acyltransferase family members
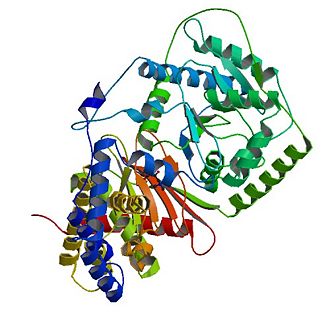
Overall structure of Carnitine acetyltransferase
|
The tertiary structure of CAT consists of 20 α-helices (α1-α20) and 16 β-strands (named β1-β16) which are arranged into two equally sized domains (N and C domains ).