User:Kristen Russo/Sandbox2 1N25
From Proteopedia
|
Contents |
Introduction
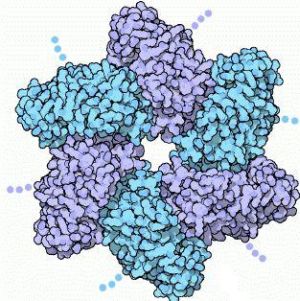
Large T-antigen (LTag) is a viral oncoprotein derived from the polyomavirus SV40 (Simian virus 40). SV40 was first identified in the cultures of Asian rhesus monkeys in 1960. SV40 infects primate cells by forcing its way into the cells and releasing its DNA. Once inside, the DNA replicates and packages it in new viral capsids. SV40 uses the T-antigen protein to control both of these processes. There are three functional parts to the T-antigen. The first part is the LTag , which forms a six-fold ring by assembling with several other copies of the protein. The hole in the middle encircles a DNA double helix.
The other two functional parts of the T-antigen are: the central domain which has a small patch and anchors the T-antigen to the SV40 genome by binding to the regulatory region, and the third domain which interacts with cellular proteins, therefore directing the different stages of the viral life cycle. There are 12 copies of the T-antigen protein which forms a long tube around the DNA. Although there are three parts to the T-antigen, LTag is the fragment that seems to be associated with the formation of cancer. LTag is a member of the helicase family III, and has been known to hinder the function of cellular tumor suppressors, such as p53. LTag targets and inhibits p53 function, which ends up deregulating cellular growth control.
LTag
How does LTag inhibit p53?
LTag forms a complex with p53 using its helicase domain (residues 251-677). The complex is composed of one LTag hexamer, binding with one monomeric p53 (which is 6 p53s). LTag alpha helix h15 binds to the p53 region 1. This makes one assymetric unit, which are arranged in 2 copies. The p53 region 1 may play a significant role when bound to DNA, in stabilizing p53 tetramer, so the binding of LTag may actually interefere with the tetramerizationof p53.