OspA L03 Group2
From Proteopedia
Contents |
Introduction
|
Lyme disease has been affecting many individuals year around developing chronic arthritis, neurologic and cardiac abnormalities, and skin lesions (Burgdorferi, 1982). The bacterial vector, Borrelia burgdorferi is spread through the bite and feeding of ticks bringing initial severe skin lesions, erythema chronicum migrans within 10-12 weeks of infestation (Burgdorferi, 1982). Studies were first conducted through New Zealand white rabbits (Burgdorferi, 1982) through the use of indirect immunofluoresence.
Outer surface protein A, or OspA is found to inhibit bacterial transmission (Ding, 2000). Outer surface protein A, or OspA was found to initiate an immune response that contributed towards the development of Lyme disease vaccinations. It prevented the transmission of Borrelia burgdorferi, the causative bacterial agent of Lyme disease after the attachment of an infected tick (Ding, 2000). Once entered the host through the skin or blood stream, Borrelia burgdorferi downregulated and suppressed OspA to minimize all of the host’s immunogenic characteristics (Rupprecht, 2008). When OspA on the spirochete migrated to an inflammatory environment, it induced apoptosis on the bacteria through the activation of B-cells (Rupprecht, 2008). Only OspA positive in borrelia bacteria were unable to establish an infection compared to OspA negative bacteria successfully hosted in studies of mice (Rupprecht, 2008). OspA known as a potent stimulator of neutrophils was able to kill the pathogen and attract leukocytes allowing the release of proinflammatory cytokines in a host (Ruprecht, 2008) and avoid contracting Lyme disease as it affects the heart, joints, and nervous system (Rupprecht, 2008). The reactive LA-2 antibody was found to serve as an important epitope of Osp-A binding (Ding, 2000) towards developing vaccinations. The free state of the 3D model exposed the C-terminal where there were 49 residues from the “three loops” involved that significantly affected by LA-2 binding, through findings from NMR and crystallization (Ding, 2000).
About OspA
Structure
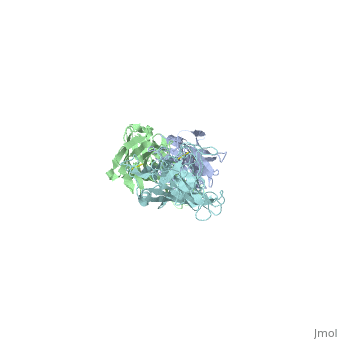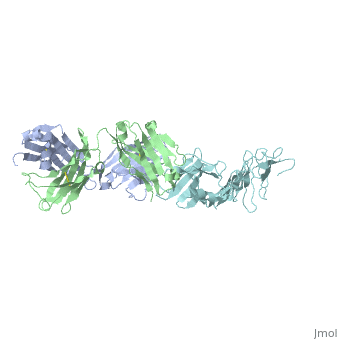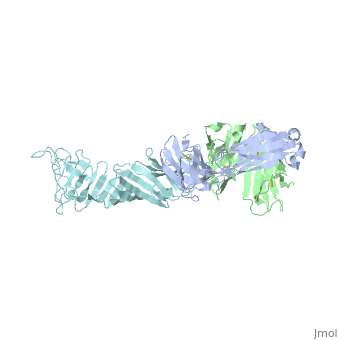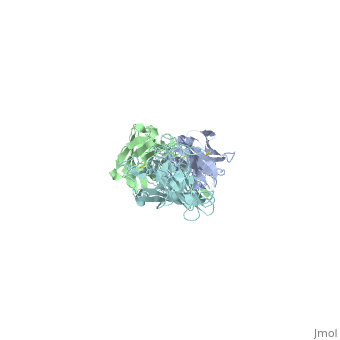
The reactive LA-2 antibody was found to serve as an important epitope of Osp-A binding (Ding, 2000) towards developing vaccinations. The protein obtained from PDB was dissected to isolate and concentrate the F chain from the molecule of OspA from the crystallized structure, since the structure was symmetric. The free state of the 3D model exposed the C-terminal where there were 49 residues from the “three loops” involved that significantly affected by LA-2 binding, through findings from NMR and crystallization (Ding, 2000). Residues 207 and 227 from “loop 1” were excluded from analysis because of the peak overlap (Ding, 2000). Residues 203 to 220 in “loop1” were represented by pale green, residues 224 to 233 in “loop 2” were colored purple and residues 246 to 257 in “loop 3” were colored medium slate blue. The cool coloring of the residues shows the location of LA-2’s direct contact on the “3 loops”. Primary colors were used to represent Ala208 as red and Ala215 as blue in spacefills for the primary or initial identification of the LA-2 epitope on the beta-strands. The coloration of resides are all at one end, C-terminal of the isolated OspA molecule showing the side where LA-2 binds. The rest of the model were beta-sheets that were left yellow, as the neutral color, and the only alpha-helix was colored pink, to show the general overall structure of 21 anti-parrallel beta-strands to 1 alpha-helix (Ding, 2000).
Vaccination (La-2)
Side Effects
Comparing OspA , OspB , OspC
References:- ↑ Ding W, Huang X, Yang X, Dunn JJ, Luft BJ, Koide S, Lawson CL. Structural identification of a key protective B-cell epitope in Lyme disease antigen OspA. J Mol Biol. 2000 Oct 6;302(5):1153-64. PMID:11183781 doi:10.1006/jmbi.2000.4119
- ↑ Rupprecht TA, Koedel U, Fingerle V, Pfister HW. The pathogenesis of lyme neuroborreliosis: from infection to inflammation. Mol Med. 2008 Mar-Apr;14(3-4):205-12. PMID:18097481 doi:10.2119/2007-00091.Rupprecht