G09SecL04Tpc2
From Proteopedia
Outer Surface Protein A (OspA) is a major lipoprotein on the borrelia burgdorferi spirochete which is a causative agent of Lyme Disease. The three loops in the C-terminus define the OspA antigen-antibody complex. The interaction between the loops and the LA-2 (antibody) forms the basis for OspA vaccination. Along with other surface proteins, OspA has many important functions that are associated with Lyme Disease such as late stage neurological disorders including acute Lyme neuroborreliosis.
Contents |
Outer Surface Protein A (OspA)
|
Introduction to Lyme Disease
Lyme disease is the most common tick-borne disease in North American hemisphere. Responsible for transmission is a tick vector from the genus of Ixodidae. There are different bacterial strains of borrelia including B.afzellii and B.garinii being very prevalent throughout the European continent; other known strains such as B.dutonii and B. recurrentis have been discovered within the past few decades. The most common strain in the United States is borrelia burgdorgeri sensu stricto, and this treponema-like spirochete was discovered to be the causative agent of Lyme Disease discovered by Dr. William Burgdorferi in 1982 along with several other colleagues.
Besides OspA, there are other outer surface proteins, including OspB, OspC, and Vls, which are very important for the transmission of Lyme Disease from the tick to the host, as well as establishing the infection via bloodstream disseminating throughout the host body. Early symptoms of Lyme Disease include skin lesions and rashes that have a characteristic bulls-eye appearance known as erythema chronicum migrans (ECM). It is important to note that ECM can be used for early diagnosis of Lyme Disease prior to awaiting more accurate laboratory tests.
Upon infection, OspA is immediately downregulated or can even be turned off. Because of this, vaccinations for OspA are virtually ineffective once spirochetes have entered the host. Downregulation also makes OspA harder to detect; OspA is usually upregulated in the late stages of the disease and leads to more serious inflammatory disorders in the central nervous system.
Structure
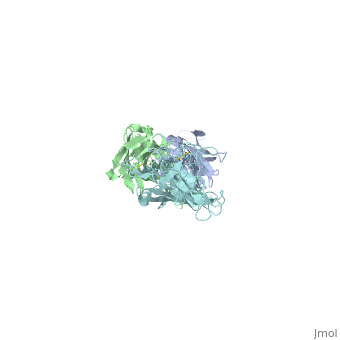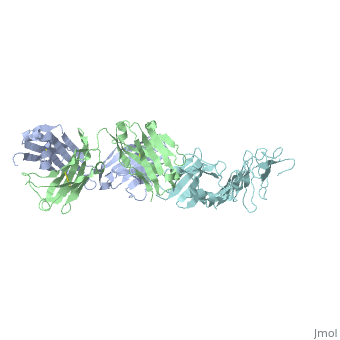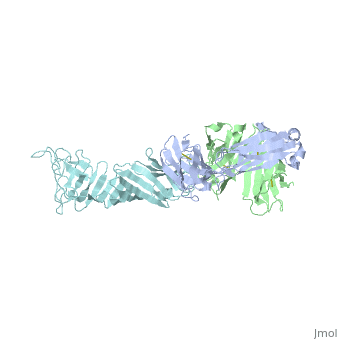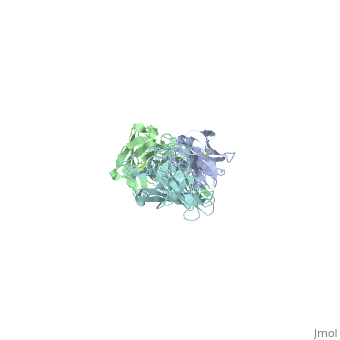
Outer Surface ProteinA has approximately 270 amino acid residues. In terms of secondary structure, OspA is made up of 21 anti-parallel single layer beta strands and one alpha helix. OspA has three major components to its structure: an n-terminal sandwich, a central sheet, and a c-terminal barrel domains. The n-terminal is often termed as a sandwich due to its jumbled formation of amino acid residues. Most of the beta strands lie on the central sheet. Lastly, the c-terminal is called barrel domains because the three primary loops connect to both sides of the central beta sheet forming a barrel or a hole in the middle. This can be seen by rotating the molecule.
The c-terminal is heavily involved in the antigen:antibody complex unlike the n-terminal. The c-terminal houses a protruding ridge of three loops: loop 1 contains residues 203-220; loop 2 contains residues 224-233; loop 3 contains residues 246-257. These loops define the LA-2 (antibody) epitope. More specifically, the loops indicate where the antibody binds to the antigen. OspA is definitely a unique lipoprotein. It’s elongated fold and protruding ridge gives the surface protein a high degree of mobility and surface exposure, especially Loop 1 in the c-terminus.
The Elusiveness of OspA
References
1. Burgdorfer W, Barbour A, Hayes S, Benach J, Grundwaldt E, and Davis J. 1982. Lyme Disease–A Tick-Borne Spirochetosis? Science. 216(4): 1317-1919.
2. Wormser GP, Dattwyler RJ, Shapiro ED, et al. (November 2006). "The clinical assessment, treatment, and prevention of Lyme disease, Human Granulocytic Anaplasmosis, and Babesiosis: Clinical Practice Guidelines by the Infectious Diseases Society of America". Clin. Infect. Dis. 43 (9): 1089–134.
3. Ding W, Huang X, Yang X, Dunn J, Luft B, Koide S, and Lawson C. 2000. Structural Identification of a Key Prospective B-cell Epitope in Lyme Disease Antigen OspA. Journal of Molecular Biology. 302(5): 1153-1164.
4. Rupprecht T, Koedel U, Fingerle V, and Pfister H. 2008. The Pathogenesis of Lyme Neurborreliosis: From Infection to Inflammation. Molecular Medicine. 14 (3): 205-212.