Sandbox 38
From Proteopedia
| Please do NOT make changes to this Sandbox. Sandboxes 30-60 are reserved for use by Biochemistry 410 & 412 at Messiah College taught by Dr. Hannah Tims during Fall 2012 and Spring 2013. |
|
Contents |
Papain
Introduction
Papain (EC 3.4.22.2) is a cysteine protease derived from the papaya plant. This protein consists of 212 amino acids that contribute to a single polypeptide chain with a molecular weight of 23.4 kD. Like other proteins, Papain contains a . The red end of the protein indicates the 3' Carbon end, while the blue end indicates the 5' Nitrogen terminal. The varying colors in between are merely used to show the progression between the two terminals. The of this molecule consists of 25% alpha helices, seen as pink "rockets", and 21% beta sheets, seen as orange "planks" . Since papain is a protease, it is able to hydrolyze the peptide bonds of leucine and glycine, however it ideally cleaves residues that are followed by a large hydrophobic residue. Traditionally, Papain has be used as a commercial meat tenderizer. This enzyme also has some medicinal uses, as it has be been used in wound debridement, and is occasionally administered as a dietary supplement. This enzyme is able to aid in digestive problems, as it is able to break down food so it is easier to digest.
History:
Papain was discovered during the colonial period in Congo, as the native inhabitants discovered that wrapping their elephant meat in papaya leaves helped to tenderize the meat. While they did not know the direct cause, this was when the proteolytic enzyme was first discovered. Papain was originally explored in 1973 by G.C. Roy, however the active binding site of this enzyme was first discovered by Drenth et al., through the crystallographic analysis of the enzymes structure.
Function:
Composition of Papain:
Papain consists primarily of carbon, nitrogen, sulfur, and oxygen. The can be view, as carbon is labled gray, oxygen red, nitrogen blue, and sulfur yellow. There are three that contribute to the structure of Papain. These bonds are indicated by their yellow color, as they link particular cysteine residues. Throughout this enzyme, there are specific charged residues, indicating the most polar portions of the molecule. These can be visualized, as the cationic residues are blue in color, while the anionic residues are red. Partially charged residues are simply lighter in color. It is interesting to note that nearly all of these charged residues are located on the outer portion of the molecule in order to interact with the surrounding environment.
Hydrophobicity and Hydrophilicity:
These charged residues contribute to the overall and regions of the protein. This results is particular hydrophobic (water hating) and hydrophilic (water loving) portions of the enzyme. These are huge factors in determining the tertiary structure of the protein. As mentioned before, the charged, hydrophilic, portions of the enzyme are primarily located on the out portion of the molecule. The polar amino acids are indicated by a purple color, while the hydrophobic residues are gray.
Ligands of Papain
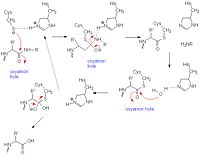
Papain binds to an abundance of , as they are the molecules colored green. Since methanol was used as a solvent in the crystallization of Papain, many of these molecules surround the protein. These ligands interact with Papain through various , which are distinctly colored blue. are also able to surround the molecule and form hydrogen bonds as well. A pseudosubstrate that is able to mimic the actual substrate and therefore inhibit Papain is called Leupeptin. The (PDC ID: 1POP), clearly indicated by the blue Nitrogen of the substrate, indicates that the inhibitors carbonyl carbon is covalently bound to the Cys-25 sulfer atom of papain and is organized in a tetrahedral manner. The carbonyl oxygen atom of the inhibitor is not only able to face the oxyanion hole, it is also able to form hydrogen bond contacts with Gln-19 and Cys-25.
References:
