Structures and Interactions
HER2
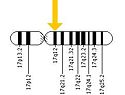
ERBB2 is located on the long arm of human chromosome 17 (17q12). This gene codes for the protein HER2 and is known as a proto-oncogene due to its ability to become an oncogene from an over-expression.
The HER family is composed of three domains: an further divided into four sub-domains composed of about 630 amino acids, a composed of a single membrane-spanning region, and an composed of a tyrosine kinase. The four sub-domains of the extracellular region include:
- sub-domain I
- sub-domain II
- sub-domain III
- sub-domain IV
In EGFR, HER3, and HER4 sub-domains I and III remain in a “closed” conformation until a ligand binds. Sub-domain II remains hidden making contact with sub-domain IV. The interaction between sub-domain II and IV can be temporarily broken in order for the receptor to become available for a ligand to bind. Although unsure, evidence suggests that the ligand binds to sub-domain III and/or sub-domain IV; also, there’s some evidence of ligand-binding to sub-domain I. Once ligand-binding is initiated, the receptor experiences a conformational change allowing sub-domains I and III to become in contact resulting in the “open” conformation. This open conformation exposes sub-domain II which allows for dimerization between receptors. Although some evidence suggests that sub-domain IV is necessary for ligand-binding, stabilizing the extracellular domain, and locking the receptor in an open conformation, the exact function is still unknown. Once the dimerization between two receptor tyrosine kinases takes place, a cross-phosphorylation reaction occurs following the activation of certain cell signaling pathways. These pathways include:
- mitogen-activated protein kinase (MAPK)
- phosphoinositide 3-kinase (PI3K/Akt)
- signal transducer and activator of transcription (STAT)
- phospholipase C
- protein kinase C (PKC)
HER2 structure differs in that sub-domains I and III are in constant contact resulting in a permanent open conformation. Sub-domains I and III in HER2 are stabilized by core hydrophobic residues surrounded by hydrophilic contacts facilitating the stabilization of this interaction. This fixed conformation allows the receptor to act in a ligand-independent manner and permits the access of sub-domain II for dimerization. Perhaps this may explain why a high-affinity ligand for HER2 has not yet been recognized. The absence of sub-domain II and IV contact can be explained by the mutations of Gly 563 and His 565 found in the other members of the HER family to Pro and Phe respectively. Since sub-domain II is always available for dimerization it is free to form heterodimers with any of the other epidermal growth factor receptors; it does not form a homodimer. Asp 285 is the only residue in sub-domain II not conserved between EGFR and HER2. Leu replaces Asp 285 in HER2 and can explain why HER2 does not form homodimers. Once dimerized, HER2 activates the MAPK pathway, which in turn initiates cell proliferation, migration, differentiation, and angiogenesis.
HER2 also has another potentially harmful ability. The juxtamembrane region of the extracellular domain is prone to proteolytic cleavage resulting in a protein called p95HER2. p95HER2 is the form of HER2 and has been shown to have an increased ability to cross-phosphorylate with the other receptors of the HER family and has increased cellular signaling capabilities.
One combination of epidermal growth factor receptors has the potential to be a potent inducer of tumorigenesis. HER2:HER3 combination can be especially detrimental to a patient exhibiting over-expression of HER2. While HER2 activates a cell proliferation pathway, HER3 is the only receptor that can directly activate the PI3K pathway. The PI3K pathway recruits its messengers (Akt and mTOR) that allows for the activation of cell survival mechanisms and helps the cell resist apoptosis. This combination can become deadly resulting in metastasized cancer.
Herceptin
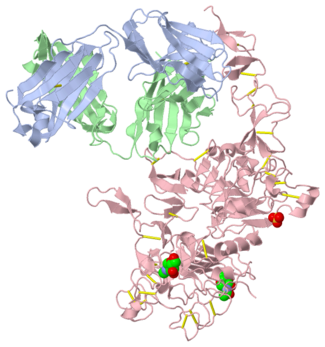
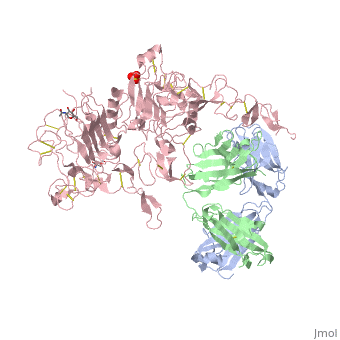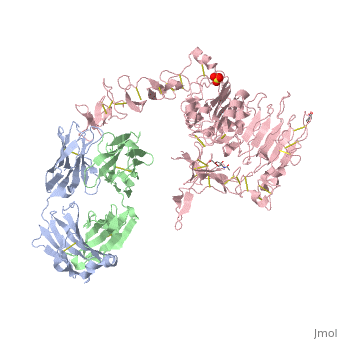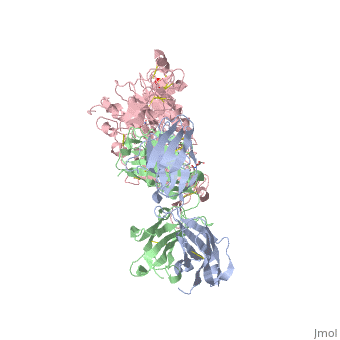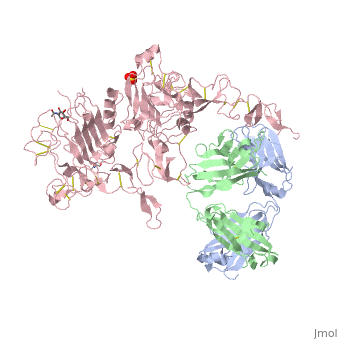
Herceptin is a monoclonal antibody proven to be a clinically effective treatment in fighting HER2+ breast cancer. There is also some clinical evidence suggesting that Herceptin enhances the effects of chemotherapy. Herceptin binds to the juxtamembrane region of HER2 on the C-terminal portion of sub-domain IV. The interaction formed by Herceptin and HER2 is mediated by three regions on HER2 that form three loops: residues 557-561 (loop 1), 570-573 (loop 2), and 593-603 (loop 3). Loops 1 and 3 are formed primarily by electrostatic interactions and loop 2 is formed by hydrophobic contacts. Herceptin acts by four different mechanisms of action:
- activation of antibody-dependent cellular cytotoxicity
- prevention of formation of p95HER2
- inhibition of cell proliferation
- inhibition of angiogenesis
When Herceptin binds to HER2 it is able to flag these cells for destruction. This works by
attracting lymphocytes to these HER2+ cells. Lymphocytes are then stimulated to release substances that cause cellular apoptosis.
An added significant mechanism of action of Herceptin is the prevention of the formation of p95HER2. By binding to the juxtamembrane region Herceptin sterically hinders any cleavage of the site ultimately preventing the formation of the highly active p95HER2.
The next two mechanisms of action for Herceptin involve intracellular inhibition. By the binding of Herceptin to the extracellular region of HER2 Herceptin is able to prevent downstream activation of cell signals that induce cell proliferation and angiogenesis. Repression of the proangiogenic factors Herceptin is also able to induce antiangiogenic factors. By inhibiting these intracellular processes Herceptin prevents any further blood vessels developing towards the tumor and prevents the tumor from growing any larger.
One limitation with this therapy is that it does not prevent HER2 from dimerizing with HER3. This makes it possible for the activation of the PI3K pathway which increases the ability of the cell to survive. Further therapies targeting the prevention of HER2 to dimerize with other members of the HER family will be necessary for future investigational studies.