Sandbox Reserved 646
From Proteopedia
| This Sandbox is Reserved from 30/08/2012, through 01/02/2013 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 636 through Sandbox Reserved 685. | ||||||
To get started:
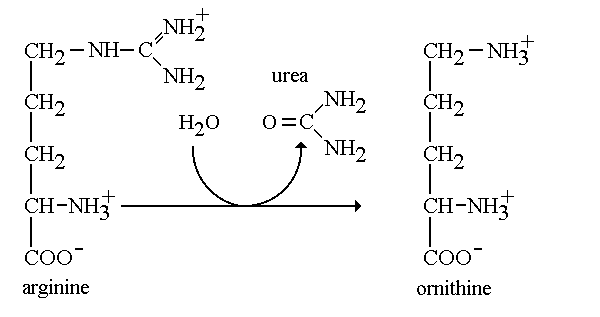
More help: Help:Editing For more help, look at this link: http://proteopedia.org/w/Help:Getting_Started_in_Proteopedia ArginaseArginase is an enzyme found within the important urea cycle. Arginase is the final enzyme that allows the Urea cycle to complete its fifth and last step. Arginase takes on two distinct forms: Class I and II. The grouping into classes refers to the catalytic activity that the enzyme is capable of performing. In addition, each class are encoded by a different gene. Arginase I (arg1) is found in the cytoplasm of liver cells functioning in the urea cycle, while arginase II (arg2) is found in the mitochondria and functions in the kidney. In interest of our studies, we will refer to arginase I as arginase. Arginase belongs to the enzymatic class hydrolase. Arginase functions by cleaving the amino acid arginine with water to produce urea. When one studies enzymes, he pays careful attention the it’s specific activity. Specific activity quantifies the amount of product formed by the enzyme in reference to a given time per concentration of a protein. Arginase is known to have high specific activity and functions to produce a good amount of urea.[1] In addition, this enzyme allows the regeneration of ornithine which is the final product of the urea cycle. When there is a deficiency in the arg1 gene it is understood that one is undergoing an urea cycle disorder. In people with arginase deficiency, arginase is either missing or harmed, therefore arginine is not metabolized correctly. In lieu of this, it is impossible for urea to be formed, leaving the excess nitrogen to accumulate in the blood in the form of ammonia. Which leads to serious problems in the body.[2] MechanismArginine + H2O → Ornithine + Urea The mechanism regarding arginase is highly important in the production of urea through the urea cycle. During the final step of the urea cycle, the amino acid arginine is present and needs to be cleaved in order for urea to be produced. Arginine is hydrolyzed and cleaved with the hydroxyl at the end of the amino group. We refer to this group as a guanidium end. The guanidium group is positively charged and has a high pKa. Therefore, it is willing to donate a proton since it has a hydrogen to give. First, the ligand will ionize the water to form hydroxide. The hydroxide now attacks the guanidine carbon and protons are transferred from the hydroxide to the substrate bridging. In the third and final step protons are transferred through a proton shuffle to create ornithine and urea. Afterwards, the urea is excreted through urine. Leaving the excess ornithine to be recycled through the final step in the cycle to react with citrulline and eliminate ammonia from the body. [3] Structure
|