Sandbox Reserved 707
From Proteopedia
The complex of wild type B-RAF and BAY439006
Contents |
Introduction
The B-RAF Protein belongs to a large family of protein kinases that have a very important role on different aspects of cellular biology and biomedical sciences. The human protein kinase family consists of 518 genes, making it one of the largest gene families. Based upon the phosphorylation of the hydroxyl group these proteins are classified on 3 groups: protein serine threonine kinase (385 members), protein tyrosine kinase (90 members) and tyrosine kinase like proteins (43 members). A-RAF, B-RAF and C-RAF proteins are a family of serine threonine kinase proteins that participate in the RAS-RAF-MEK-ERK signal transduction cascade. The general reaction they catalyse is:
This cascade participates in the regulation of several cellular mechanisms like: apoptosis, cell cycle progression, differentiation, proliferation and transformation to the cancerous state in response to growth factors, cytokines and hormones. First discovered in 1938 as a retroviral oncogene B-RAF plays a key role on all studies concerning cancer therapies and other biomedical applications.
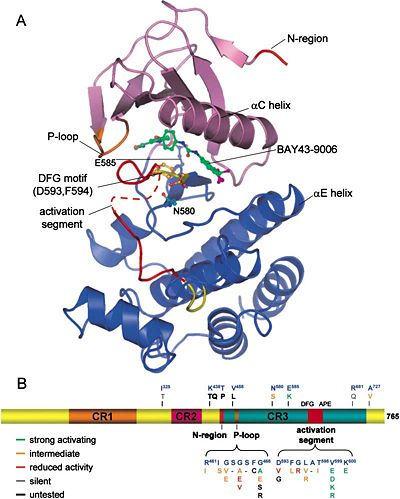
B-RAF Structure[1]
|
As we distinguished before, there are 3 types of RAF proteins: A-RAF, B-RAF and C-RAF. All of them share 3 much conserved regions (CR): CR1, CR2 and CR3.
CR1 is composed of a RAS binding domain (RBD) and a cysteine rich domain (CRD) which can bind 2 zinc ions (zinc finger domain). It is possible for the CR1 to interact with the RAS and with the membrane phospholipids (PH domain).
CR2 is a serine threonine rich domain. When a serine is phosphorylated this domain can bind a regulatory protein that can bind the C-terminal region in the same time. Binding of 14-3-3 (C-terminal region) to this phosphorylated serine is inhibitory for the enzyme.
CR3 is the protein kinase domain on the C-terminal region. We can find just after this kinase domain a stimulatory 14-3-3 binding site for other regulatory proteins.
As all RAF Proteins, B-RAF has the characteristic small N-terminal lobe and the large C-terminal lobe.
The small lobe is constituted of an antiparallel β-shift that anchors and orient the ATP. It contains a glycine rich ATP phosphate binding loop, called P-loop. The large lobe interacts with the substrate that in our case is MEK1/2 who needs to be phosphorylated to be active. The catalytic site is just between the two lobes. These 2 lobes can move relative to each other, opening or closing the cleft. This has 2 major consequences on the functioning of this enzyme:
1. The open form allows access of ATP and release of ADP from the active site.
2. The closed form brings the residues of the substrate into the active site.
On the other hand each lobe has her proper polypeptide segment that can change conformation from active to inactive and vice versa.
1. In the small lobe, this segment is a α-helix, which is called αC-helix. The αC-helix rotates and translates with respect to the rest of the lobe, making or breaking part of the active site.
2. In the large lobe, the activation segment can make or break part of the ATP binding site.
The activation segment of each protein kinase has a specific domain that begins with a DFG amino acid sequence that can easily change its conformation (active or inactive conformation). In the inactive conformation the phenylalanine side chain occupies the ATP binding pocket and the aspartate side chain faces away from the active site. This is the DFG Aspartate Out Conformation. In the active conformation, the phenylalanine side chain is rotated out of the ATP binding pocket and the Aspartate side chain can now face the ATP binding pocket and form coordinated links with the Mg²⁺. This is called the DFG Aspartate In Conformation. The activation segment can be phosphorylated by members of the same protein kinase family or by other protein kinases.
A gatekeeper residue separates the adenine binding site from the hydrophobic pocket. Mutation on this residue can prevent the binding of kinase inhibitory drugs (the replacement of a threonine by a methionine for example).
It is very important to distinguish conserved amino acid residue signatures that constitute the catalytic core of the B-RAF Kinase. The catalytic properties are done by the KDD (Lys578-Asp576-Asp594) Motif and the activation segment starts in general with a DFG and ends with APE. But how the catalytic reaction is generated?
An invariant lysine (Lys578 in B-RAF) forms salt bridges with the gamma phosphate of the ATP. Asp576, which is a base in the catalytic loop, orients the seryl or threonyl group of the substrate protein and takes the proton of the hydroxyl group, facilitating the attack of oxygen on the gamma phosphorus atom of MgATP. Asp594 binds Mg²⁺ which coordinates the beta and gamma phosphates of ATP.
This is a table of the important residues of B-RAF:
RBD: 155-227 CRD: 234-280 CR1: 150-290 CR2: 360-375 CR3, protein kinase domain: 451-717 Glycine Rich Loop: 463-471 14-3-3 binding site: S365-S729 Gatekeeper Residue: T529 HRD: 574-576 K of KDD: 578 DFG: 594-596 Activation segment phosphorylation sites: T599-S602 End of Activation segment: APE 621-623 No. of residues: 766 Molecular Weight (kDa): 84.4 UniProtKB accession No.: P15056
Regulation
The regulation of RAF Kinases involves protein-protein interactions, phosphorylations, dephosphorylations and conformational changes.
RAF kinases participate in the RAS-RAF-MEK-ERK signal transduction cascade, which is sometimes denoted as the mitogen-activated protein kinase (MAPK) cascade. Growth factors bind to receptor tyrosine kinases (RTKs), resulting in RAS activation. RAF proteins are one of a family of effector proteins activated by RAS, and they in turn stimulate the activation of MEK, which subsequently stimulates ERK activity. ERK phosphorylates both cytosolic and nuclear proteins, thereby mediating the cellular responses cells make when this pathway is activated.
Under non stimulatory conditions a serine of CR2 domain and another near the C-terminus are phosphorylated and are bound to 14-3-3 domain. To activate RAF Kinases, RAS-GTP has to interact with the RDB Domain. This is necessary but not enough to activate the RAF Kinase.
One characteristic that distinguish B-RAF from A or C-RAF is the presence of Asp448 and Asp449 on the N-terminal region, which bear negative charges. Phosphorylation of Thr599 and Ser602 in the activation segment is essential for B-RAF activation. Another residue is crucial for the activation of this enzyme as well: the phosphoserine 579 of the catalytic loop. That’s why the basal activity of B-RAF is greater than A or C-RAF.
Although X-ray structures of B-RAF kinase domains were said to be monomeric, each of the six structures in the protein data bank (2009), including B-RAF (V600E), showed that the asymmetric unit of the crystal contains two kinase domains that interact in a unique side-to-side fashion involving the N-lobe. This side-to-side dimerization involves the αC helix, an important determinant of active and inactive conformations. Based upon ultracentrifugation studies, it is demonstrated that the catalytic domain of human B-RAF forms homodimers. Arg509 of B-RAF occurs in the side-to-side interface, and these investigators found that the Arg509 His mutant exists as a monomer. But B-RAF can form heterodimers too. Ser621 and residues in the αC helix of C-RAF participate in dimer formation. B-RAF–C-RAF heterodimers are more active than either homodimer. Phosphorylation of B-RAF at Thr753 as catalyzed by ERK destabilizes heterodimer formation with C-RAF and decreases kinase activity.
Other Inhibitors
drugs and inhibitor paradox
Applications in Medicine
External Ressources
References
- ↑ Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004 Mar 19;116(6):855-67. PMID:15035987
Protreopedia Page Contributors and Editors
Kostika Sofroni and Gilles Dupouy