1wco
From Proteopedia
|
THE SOLUTION STRUCTURE OF THE NISIN-LIPID II COMPLEX
Overview
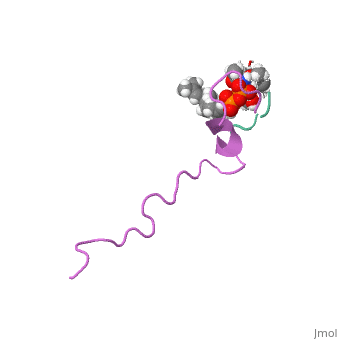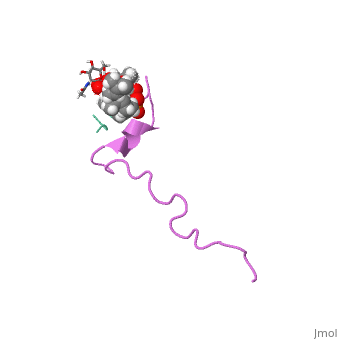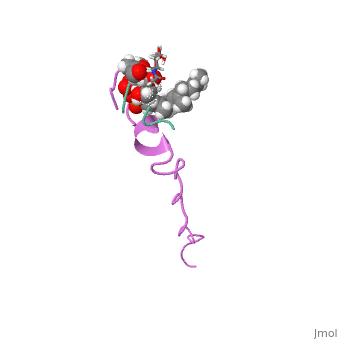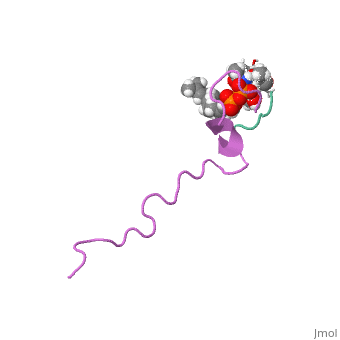
The emerging antibiotics-resistance problem has underlined the urgent need, for novel antimicrobial agents. Lantibiotics (lanthionine-containing, antibiotics) are promising candidates to alleviate this problem. Nisin, a, member of this family, has a unique pore-forming activity against, bacteria. It binds to lipid II, the essential precursor of cell wall, synthesis. As a result, the membrane permeabilization activity of nisin is, increased by three orders of magnitude. Here we report the solution, structure of the complex of nisin and lipid II. The structure shows a, novel lipid II-binding motif in which the pyrophosphate moiety of lipid II, is primarily coordinated by the N-terminal backbone amides of nisin via, intermolecular hydrogen bonds. This cage structure provides a rationale, for the conservation of the lanthionine rings among several lipid, II-binding lantibiotics. The structure of the pyrophosphate cage offers a, template for structure-based design of novel antibiotics.
About this Structure
1WCO is a Single protein structure of sequence from Lactococcus lactis and Monarthropalpus flavus with , and as ligands. This structure superseeds the now removed PDB entry 1UZT. Full crystallographic information is available from OCA.
Reference
The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics., Hsu ST, Breukink E, Tischenko E, Lutters MA, de Kruijff B, Kaptein R, Bonvin AM, van Nuland NA, Nat Struct Mol Biol. 2004 Oct;11(10):963-7. Epub 2004 Sep 12. PMID:15361862
Page seeded by OCA on Wed Jan 23 15:14:15 2008