Superoxide Dismutase
From Proteopedia
|
Superoxide dismutase (SOD) are a group of antioxidant enzymes which catalyze the dismutation of superoxide to oxygen and hydrogen peroxide. SODs are critical antioxidative proteins, protecting the host from oxidative damage. See also Molecular Playground/ Copper-Zinc Superoxide Dismutase.
Contents |
Types of SOD:
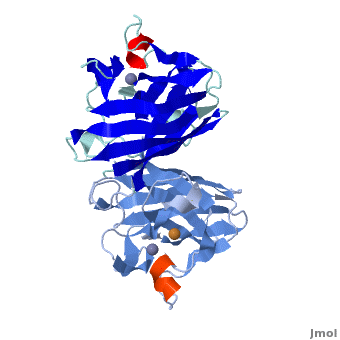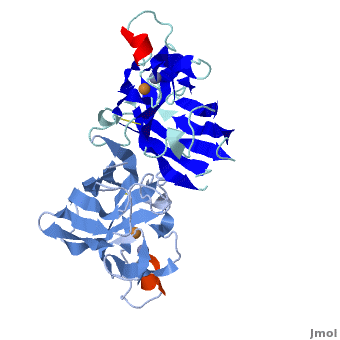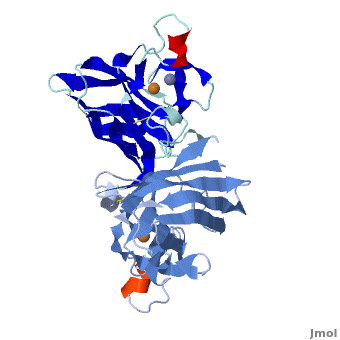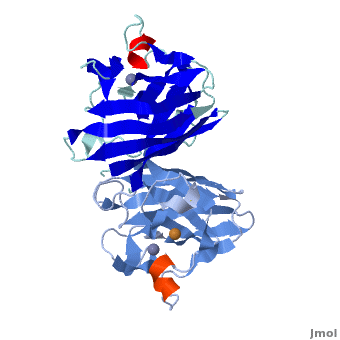
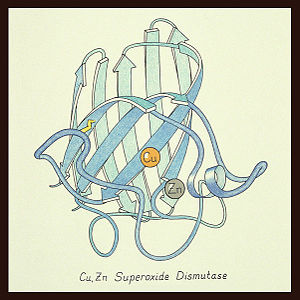
uses metals as cofactors and are named accordingly: Cu-Zn-SOD, Cu-SOD, Fe-SOD, Mn-SOD. The Cu-Zn-SOD is most commonly found in eukaryotes, including humans, while Fe-SOD and Mn-SOD are found in prokaryotes. Mn-SOD is also found in eukaryotes (see 2adq and Nitrotyrosine). The 2D and 3D images above are of a Human Cu-Zn-SOD with metal ions visible.
Structure:
|
The function of was first determined by Irwin Fridovich and Joe McCord in 1969[1][2], with the structure of bovine SOD solved soon after by David and Jane Richardson et al. [3][4] The typical SOD structure is an 8-stranded “Greek Key” beta-barrel with an active site buried between the and . While the typical biological unit for SOD is either dimer or tetramer, monomer forms exist. The Image at the left, drawn by Jane Richardson, illustrates the Greek-Key structure and position of the metal cations.
Biochemistry
Superoxide is a highly reactive oxygen species and is a major source of oxidative stress in the body, reacting with cellular targets, often causing oxidative damage. [5] SOD protects the body by safely metabolizing the superoxide into unreactive oxygen and hydrogen peroxide. Experiments conducted with knockout mice unable to produce SOD develop widespread oxidative damage and hepatocarcinogenesis and exhibit a greatly reduced lifespan. [6] In humans, mutations to SOD1, one of 3 types found in the human body, can cause familial ALS, a motor neuron disease better known as Lou Gherig’s Disease [7], and has also been linked to Down’s Syndrome[8].
Additional Resources
See: Copper, Zinc Superoxide Dismutase
3D Structures of SOD
Update July 2012
Metal-free apo SOD
3hog – SOD – tomato
1p7g – SOD – Pyrobaculum aerophilum
1ozt, 3gzp, 3gzq, 3ecv, 3ecw, 2gbu - hSOD (mutant) - human
3ecu, 3k91 – hSOD
1rk7 - hSOD (mutant) – NMR
3kbe – CeSOD – Caenorhabditis elegans
1t6i, 1t6q – ScSOD - Streptomyces coelicolor
3ak1 - ApSOD - Aeropyrum pernix
Cu-Zn-SOD
2jlp, 2v0a, 2c9v, 2c9u, 2c9s, 1pu0, 1hl5, 1sos, 1spd, 3kh3, 3kh4, 3re0 - hSOD +Zn+Cu - human
3h2p, 3h2q, 3h2r, 3hff, 3gqf, 2nnx, 1p1v, 1oez, 2xjl, 3qqd – hSOD (mutant) +Zn
2af2, 1kmg - hSOD (mutant) +Zn – NMR
1l3n, 1dsw, 1ba9 – hSOD (mutant) +Zn+Cu - NMR
2wyz - hSOD (mutant) +Zn+Cu+UMP
2wz0 - hSOD (mutant) +Zn+Cu+aniline
2wz5 - hSOD (mutant) +Zn+Cu+methionine
2wz6 - hSOD (mutant) +Zn+Cu+quinazoline
2r27 - hSOD +Cu
1hl4 - hSOD +Zn
2wko, 3gzo, 2zkw, 2zkx, 2zky, 3cqp, 3cqq, 2vr6, 2vr7, 2vr8, 2gbt, 2gbv, 1uxl, 1uxm, 1ptz, 1ozu, 1n18, 1n19, 1fun, 1mfm, 1azv, 2wyt, 2xjk – hSOD (mutant) +Zn+Cu
3l9e - smSOD +Zn – silk moth
3l9y - smSOD +Zn+Cu
2wn0, 2wwn, 2wwo – YpSOD+Zn+Cu – Yersinia pseudotuberculosis
2wn1 - YpSOD+Zn+Cu+N3
2z7u, 2z7w, 2z7y, 2z7z, 2aeo, 1q0e, 1e9p, 1e9q, 1cb4, 1cbj, 1sxn, 1sxa, 1sxb, 1sxc, 1spd, 1sda, 3sod, 2sod, 2zow, 3hw7 – cSOD+Zn+Cu - cow
1cob – cSOD+Cu+Co
1sxs - cSOD +Zn+Cu+SCN
1sxz - cSOD +Zn+Cu+N3
1e9o – cSOD+Cu
3f7k - ApSOD +Zn+Cu+H2O2 – Alvinella pompejana
3f7l - ApSOD +Zn+Cu
2k4w – SeSOD (mutant) +Zn+Cu – Salmonella enterica – NMR
1eqw - SOD +Zn+Cu – Salmonella typhimurium
3ce1 - SOD +Zn+Cu – Cryptococcus liquefaciens
2q2l - SOD +Zn – Potentilla atrosanguinea
2aqm - SOD +Zn+Cu – Brucella abortus
2aps - SOD +Zn+Cu – Actinobacillus pleuropneumoniae
2aqn - NmSOD +Zn+Cu – Neisseria meningitides
2aqp, 2aqq, 2aqr, 2aqs, 2aqt – NmSOD (mutant) +Zn+Cu
1z9n - HdSOD +Zn+Cu+haem – Haemophilus ducreyi
1z9p - HdSOD +Zn+Cu
1to4, 1to5 - SOD +Zn+Cu – Schistosoma mansoni
1pzs - MtSOD +Cu – Mycobacterium tuberculosis
1oaj, 1bzo, 1yai - PlSOD +Zn+Cu – Photobacterium leiognathi
1oal, 1ib5, 1ibb, 1ibd, 1ibf, 1ibh – PlSOD (mutant) +Zn+Cu
1f18, 1f1a, 1f1d, 1b4t – ySOD (mutant) +Zn+Cu – yeast
1f1g, 1b4l, 2jcw, 1sdy - ySOD +Zn+Cu
1yaz - ySOD +Zn+Cu+N3
1jk9 – ySOD+Zn+Cu chaperone for SOD
1eso - EcSOD +Zn+Cu - Escherichia coli
1yso - EcSOD +Zn+Cu
1srd – SOD+MZn+Cu –Spinach
3gtt – mSOD+Zn – mouse
3gtv, 3ltv – mSOD/hSOD+Zn chimera
3kbf – CeSOD+Zn+Cu
3km1, 3km2 – tSOD+Zn
3mkg – SOD + Zn - tomato
3mnd – SOD + Zn + Cu – Pig tapeworm
3pu7 - tSOD + Zn + Cu
Mn-SOD
Human Mn-SOD is discussed in the article on Nitrotyrosine.
3k9s – EcSOD+H2O2
1d5n, 1vew, 3ot7 - EcSOD+Mn
1zlz, 1ixb, 1ix9, 1en4, 1en5, 1en6, 1i08, 1i0h – EcSOD (mutant)+Mn
3dc5, 3dc6 – SOD+Mn – Caenorhabditis elegans
3bfr, 1jcv, 3lsu, 3rn4 – ySOD+Mn
2qka, 2adp, 2adq, 1xdc, 1xil, 1n0j, 1luv, 1msd – hSOD+Mn
2qkc, 3c3s, 3c3t, 2p4k, 1zsp, 1zte, 1zuq, 2gds, 1szx, 1pl4, 1pm9, 1n0n, 1luw, 1ja8, 1em1, 1ap5, 1ap6, 1qnm, 1var – hSOD (mutant)+Mn
2rcv – SOD+Mn – Bacillus subtilis
1xre, 1xuq – SOD+Mn – Bacillus anthracis
2aw9, 2cdy, 2ce4, 3kky – DrSOD+Mn – Deinococcus radiodurans
2a03 – SOD+Mn+Zn – Plasmodium berghei
1jr9 - SOD +Zn+Mn – Virgibacillus halodenitrificans
1gv3 – SOD+Mn – Anabaena
1kkc – SOD+Mn – Aspergillus fumigatus
1xso – SOD+Mn – Xenopus laevis
1mng, 3mds – TtSOD+Mn – Thermus thermophilus
3evk – SOD+Mn – Pyrobaculum aerophilum
1ar4, 1ar5 – PfSOD+Mn - Propionibacterium freudenreichii< br />
3ak2 - ApSOD+Mn
Fe-SOD
3js4 – SOD+Fe – Anaplasma phagocytuphilum
1mmm – EcSOD+Fe
1y67 – DrSOD+Fe
3esf – SOD (mutant)+Fe – Trypanosoma brucei
2gpc – SOD+Fe – Trypanosoma cruzi
3h1s – SOD+Fe – Francisella tularensis
2w7w – SOD+Fe – Aliivibrio salmonicida
3cei – SOD+Fe – Helicobacter pylori
2goj, 2bpi – PfSOD+Fe – Plasmodium falciparum
2awp – SOD+ion – Plasmodium knowlesi
2bkb, 2nyb, 1za5 – EcSOD (mutant)+Fe
2cw2, 2cw3 – SOD+Fe – Perkinsus marinus
1wb7, 1wb8 – SOD (mutant)+Fe – Sulfolobus solfataricus
1unf – SOD+Fe – Vigna unguiculata
1uer, 1ues, 1qnn – SOD+Fe – Porphyromonas gingivalis
1my6 – SOD+Fe – Thermosynechococcus elongates
1ma1 – SOD+Fe – Methanothermobacter thermautotrophicus
1gn2, 1gn3, 1gn4, 1gn6 – MtSOD (mutant)+Fe
1ids – MtSOD+Fe
1dt0, 3sdp – SOD+Fe – Pseudomonas putida
1b06 – SOD+Fe – Sulfolobus acidocaldarius
1bs3 – PfSOD+Fe+F
1bsm, 1bt8 – PfSOD+Fe
1avm – PfSOD+Fe+N3
1coj – SOD+Fe – Aquifex pyrophilus
1isa, 1isb, 1isc – EcSOD+Fe
3lio, 3ljt, 3lj9, 3ljf – SOD+Fe
3ak3 - ApSOD+Fe
3tqj – SOD + Fe – Coxiella burneti
4f2n - SOD + Fe – Leishmania major
Ni-SOD
3g4x, 3g4z, 3g50, 1t6u – ScSOD (mutant)+Ni
1q0d, 1q0f, 1q0g, 1q0k, 1q0m – SOD+Ni – Streptomyces seoulensis
References
- ↑ McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049-55. PMID:5389100
- ↑ McCord JM, Fridovich I. Superoxide dismutase: the first twenty years (1968-1988). Free Radic Biol Med. 1988;5(5-6):363-9. PMID:2855736
- ↑ Richardson J, Thomas KA, Rubin BH, Richardson DC. Crystal structure of bovine Cu,Zn superoxide dismutase at 3 A resolution: chain tracing and metal ligands. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1349-53. PMID:1055410
- ↑ Tainer JA, Getzoff ED, Beem KM, Richardson JS, Richardson DC. Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J Mol Biol. 1982 Sep 15;160(2):181-217. PMID:7175933
- ↑ Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995 Dec;11(4):376-81. PMID:7493016 doi:http://dx.doi.org/10.1038/ng1295-376
- ↑ Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005 Jan 13;24(3):367-80. PMID:15531919
- ↑ Al-Chalabi A, Leigh PN. Recent advances in amyotrophic lateral sclerosis. Curr Opin Neurol. 2000 Aug;13(4):397-405. PMID:10970056
- ↑ Groner Y, Elroy-Stein O, Avraham KB, Schickler M, Knobler H, Minc-Golomb D, Bar-Peled O, Yarom R, Rotshenker S. Cell damage by excess CuZnSOD and Down's syndrome. Biomed Pharmacother. 1994;48(5-6):231-40. PMID:7999984
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Alexander Berchansky, Eric Martz, Joel L. Sussman, Jane S. Richardson, Jaime Prilusky