From Proteopedia
proteopedia linkproteopedia link
| This Sandbox is Reserved from 30/01/2013, through 30/12/2013 for use in the course "Biochemistry II" taught by Hannah Tims at the Messiah College. This reservation includes Sandbox Reserved 686 through Sandbox Reserved 700.
|
To get started:
- Click the edit this page tab at the top. Save the page after each step, then edit it again.
- Click the 3D button (when editing, above the wikitext box) to insert Jmol.
- show the Scene authoring tools, create a molecular scene, and save it. Copy the green link into the page.
- Add a description of your scene. Use the buttons above the wikitext box for bold, italics, links, headlines, etc.
More help: Help:Editing
|
Complera: Emtricitabine/rilpivirine/tenofovir
- Marketed by: Gilead Sciences and Janssen Therapeutics
- Major Indication: HIV infection
- Drug Class: Combination of nucleoside/tide reverse transcriptase inhibitor and nonnucleoside reverse transcriptase inhibitor
- Date of FDA Approval: August 2011
Rilpivirine (RPV)
RPV in the
In red are the binding pocket residues p66 (Leu-100, Lys-101, Lys-103, Val-106, Thr-107, Val-108, Val-179, Tyr-181, Tyr-188, Val-189, Gly-190, Phe-227, Trp-229, Leu- 234, and Tyr-318) and p51(Glu-138). Singh et al.
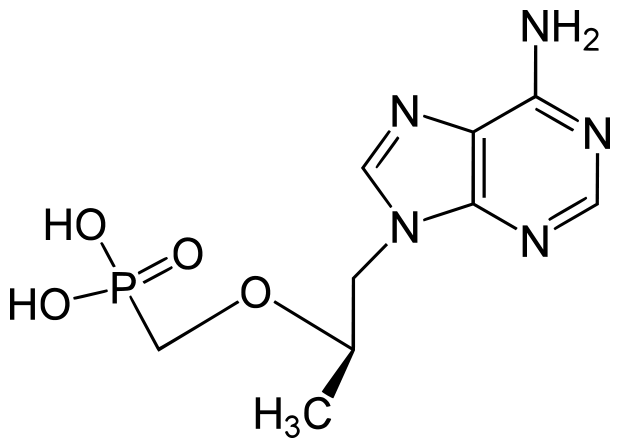
Tenofovir
Reverse Transcriptase:
Emtricitabine
Image:Emtricitabine3.png

