2h9i
From Proteopedia
|
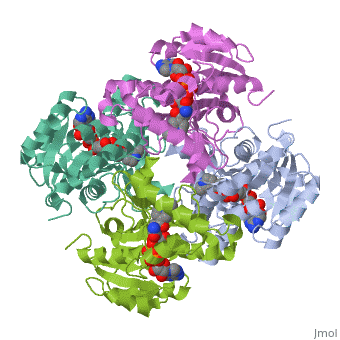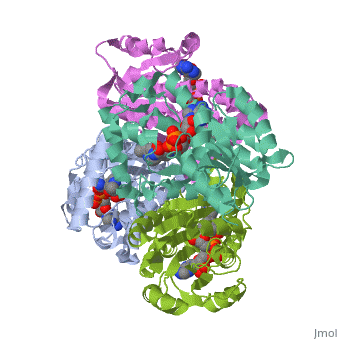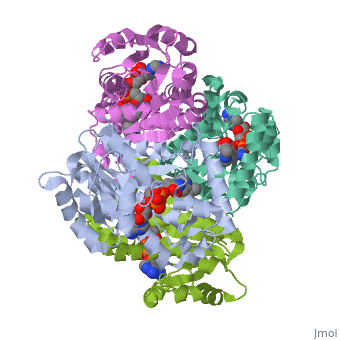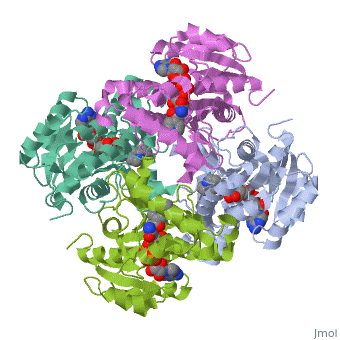
Mycobacterium tuberculosis InhA bound with ETH-NAD adduct
Overview
Thioamide drugs, ethionamide (ETH) and prothionamide (PTH), are clinically, effective in the treatment of Mycobacterium tuberculosis, M. leprae, and, M. avium complex infections. Although generally considered second-line, drugs for tuberculosis, their use has increased considerably as the number, of multidrug resistant and extensively drug resistant tuberculosis cases, continues to rise. Despite the widespread use of thioamide drugs to treat, tuberculosis and leprosy, their precise mechanisms of action remain, unknown. Using a cell-based activation method, we now have definitive, evidence that both thioamides form covalent adducts with nicotinamide, adenine dinucleotide (NAD) and that these adducts are tight-binding, inhibitors of M. tuberculosis and M. leprae InhA. The crystal structures, of the inhibited M. leprae and M. tuberculosis InhA complexes provide the, molecular details of target-drug interactions. The purified ETH-NAD and, PTH-NAD adducts both showed nanomolar Kis against M. tuberculosis and M., leprae InhA. Knowledge of the precise structures and mechanisms of action, of these drugs provides insights into designing new drugs that can, overcome drug resistance.
About this Structure
2H9I is a Single protein structure of sequence from Mycobacterium tuberculosis with EAD as ligand. Active as [acyl-carrier-protein_reductase_(NADH) Enoyl-[acyl-carrier-protein] reductase (NADH)], with EC number 1.3.1.9 Full crystallographic information is available from OCA.
Reference
Mechanism of thioamide drug action against tuberculosis and leprosy., Wang F, Langley R, Gulten G, Dover LG, Besra GS, Jacobs WR Jr, Sacchettini JC, J Exp Med. 2007 Jan 22;204(1):73-8. Epub 2007 Jan 16. PMID:17227913 [[Category: Enoyl-[acyl-carrier-protein] reductase (NADH)]]
Page seeded by OCA on Wed Nov 21 11:37:19 2007