Glycogenin
From Proteopedia
Template:STRUCTURE 3t7m Glycogenin (Glycogenin glucosyltransferase, EC 2.4.1.186) is a transferase responsible for the biosynthesis of glycogen; an important storage form of glucose in the body. It is a unique enzyme in that it is primer, substrate, catalyst, and product of its enzymatic reaction and extension process of glycogen biosynthesis. This is initiated by its ability to transfer glucose from UDP-glucose to form an oligosaccharide of glucose units that is covalently attached to itself at Tyr-194 in a multistep reaction mechanism [1]. It is placed in glycosyltransferase family 8 becuase it contains highly conserved motifs that are common to glycosyltransferases such as lipopolysaccharide glucose and galactose transferases and galactinol synthases [2].
Contents |
Structure Overview
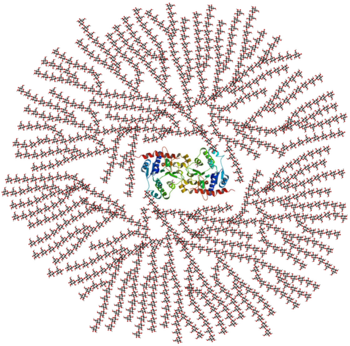
Glycogenin is found to exist in both dimeric and decameric forms. While the dimeric form has been identified as the physiological relevant active form, the attractive, star-like decameric form (pentamer of dimers) is likely a result of high enzyme concentration that is required for crystallization; the structure doesn't fit into the structural and kinetic model of self-glucosylation due to the monomeric confinement of the active sites [1]. The unit contains a single domain with a mixed containing a six-stranded and three-stranded beta-sheet; surrounded by alpha-helices in what resembles a four-layered alpha/beta sandwich around the where Biosynthesis is initiated [1]. The highly conserved DxD motif, for the coordination of the catalytic divalent cation (Mn2+), and the N-terminal beta-alpha-beta Rossman fold-like domains are both common to nucleotide-binding domains in similar glycosyltransferases [1].
Protein Function
|
UDP-alpha-D-glucose + glycogenin <-> UDP + alpha-D-glucosylglycogenin
The glycogenin binds at the Tyr-194 in and then the enzyme is primed for extension by subsequent UDP-glucose additions for glycogen formation. The glucosyltransferase activity of glycogenin catalyzes the addition of subsequent UDP-glucose monomers to form a glucose polymer roughly 7 residues long. The reaction is then joined by the enzyme glycogen synthase which continues the α-1,4-glycosidic elongation of the glucose polymers, and glycogen branching enzyme that catalyzes α-1,6-glycosidic branch formation of the glycogen. The Mn2+ cation functions as a lewis acid to stabilize the UDP leaving group and help fascilitate the transfer from the Tyr-194 to another nucleophilic intermediate acceptor, Asp-162, in a dual-step nucleophilic SN1 substitution reaction [1].
Protein Regulation
Glycogenin is a self-glucosylating protein that primes glycogen synthesis and the amount of glycogenin present contributes to the glycogen production and glucose storage capacity in an individual. The activity of glycogenin as the rate limiting enzyme in glycogen synthesis indicates that its activity must be subject to strict regulatory control [3].
Physiological Influences
Glycogenin is an important protein in terms of managing energy replenishment after glycogen depletion from prolonged physical activity. During recovery after intense exercise, glycogenin mRNA activity increases substantially in muscle to fascilitate the rapid glycogen re-synthesis by increasing the levels of glycogenin which is the backbone for the formation of proteoglycogen; remaining covalently attached to the non-reducing end in the centre of the globule that it is primes and initiates. [4]. Proteoglycogen granules are the initial, small form of glycogen that have roughly an equal ratio of protein to glucose content; maturing into macroglycogen, which is a larger highly branched storage form with a higher carbohydrate to protein ratio. The proteoglycogen pools are the dominant form because they are readily degraded during exercise and quickly re-sythesized during post exercise recovery when glycogenin levels are increased [4].
Genetics and Mutations
Glycogenin has been identified in two human isoforms. Glycogenin-1 is a 37kDa muscle isoform encoded for by the gene GYG1, whereas glycogenin-2 is the 66kDa liver isoform that is encoded by the gene GYG2 and expressed primarily in cardiac muscle [5]. Mutations of the GYG1 gene results in a loss of the autoglycosylation capabilities of glycogenin for initiating glycogen synthesis in muscle, which leads to problems such as cardiac arrhythmia and muscle weakness due to depleted or abnormal storage of glycogen in heart and skeletal muscle [5].
3D structures of glycogenin
Updated on 10-March-2013
3q4s - hGYG1 – human
1ll0, 1ll3, 3v8y – rGYG1 – rabbit
1zcu, 1zcv, 1zcy, 3usq, 3usr, 3v90 - rGYG1 (mutant)
Glycogenin complex with Mn+2 ion and UDP
3u2t - hGYG1 + Mn
3rmv – hGYG1 (mutant) + Mn + UDP
3rmw - hGYG1 (mutant) + Mn + UDP-glucose
3t7o - hGYG1 + Mn + UDP-glucose + glucose
3u2x - hGYG1 + Mn + UDP + glucose
3qvb, 3t7m, 3t7n - hGYG1 + Mn + UDP
3u2u - hGYG1 + Mn + UDP + maltotetraose
3u2v - hGYG1 + Mn + UDP + maltohexaose
3u2w - hGYG1 (mutant) + Mn + glucose
1zct, 3v8z – rGYG1 + Mn + UDP
1zdf, 1zdg, 3v91 - rGYG1 (mutant) + Mn + UDP-glucose
1ll2 - rGYG1 + Mn + UDP-glucose
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Gibbons BJ, Roach PJ, Hurley TD. Crystal structure of the autocatalytic initiator of glycogen biosynthesis, glycogenin. J Mol Biol. 2002 May 31;319(2):463-77. PMID:12051921 doi:http://dx.doi.org/10.1016/S0022-2836(02)00305-4
- ↑ Henrissat B, Davies G. Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol. 1997 Oct;7(5):637-44. PMID:9345621
- ↑ Alonso MD, Lomako J, Lomako WM, Whelan WJ. A new look at the biogenesis of glycogen. FASEB J. 1995 Sep;9(12):1126-37. PMID:7672505
- ↑ 4.0 4.1 Shearer J, Wilson RJ, Battram DS, Richter EA, Robinson DL, Bakovic M, Graham TE. Increases in glycogenin and glycogenin mRNA accompany glycogen resynthesis in human skeletal muscle. Am J Physiol Endocrinol Metab. 2005 Sep;289(3):E508-14. Epub 2005 May 3. PMID:15870102 doi:10.1152/ajpendo.00100.2005
- ↑ 5.0 5.1 Moslemi AR, Lindberg C, Nilsson J, Tajsharghi H, Andersson B, Oldfors A. Glycogenin-1 deficiency and inactivated priming of glycogen synthesis. N Engl J Med. 2010 Apr 1;362(13):1203-10. PMID:20357282 doi:10.1056/NEJMoa0900661
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, David Canner, Kim Settle