Enzyme I of the S. aureus PTS
From Proteopedia
|
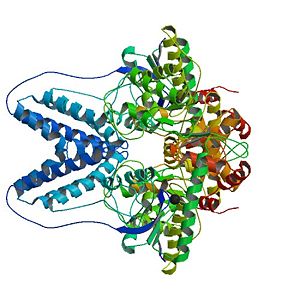
Here we report the crystal structure of Enzyme I (EI) from Staphylococcus aureus which is the first component involved in the Sugar Phosphotransferase System (PTS) reaction cascade. EI is an enzyme of 572 residues and its tertiary structure is composed of 48% α helix and of 13% β-sheet. The crystal structure was obtained with a resolution of 2,40Ǻ.
Contents |
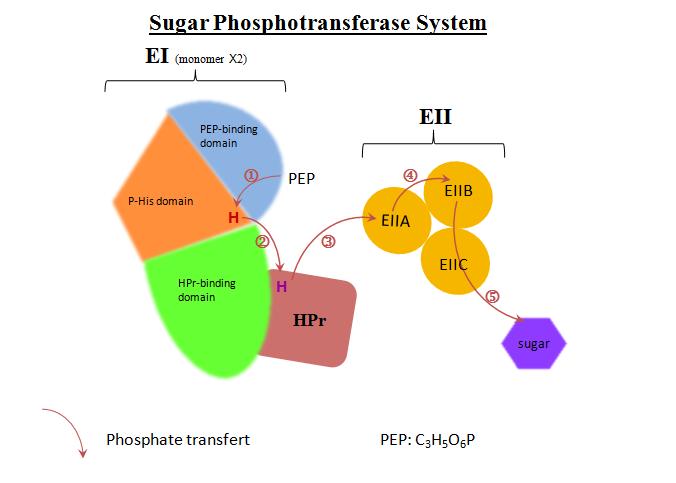
I.The Sugar Phosphotransferase System
PTS have two functions in organisms, the first one is the control of sugar consumption, the second one is the control of the carbon metabolism. PTS is composed of four phosphoproteins, whose aim is to regulate the transfer of a phosphyl group from phosphoenolpyruvate (PEP) to sugar which can then move into the cell. PEP is the phosphate stock in this system. The phosphate transfer occurs by five phosphorylation steps :
-PEP binds on the PEP binding domain of the C terminal domain of EI (EIC)
-The phosphate is carried from PEP to phospho-histidine domain (P-His) of the N terminal domain of EI (EIN) on His191.
-P-His domain transfers phosphate from PEP to the general phosphoryl carrier protein (HPr) linked to the HPr-binding subdomain of EIN.
-Enzyme II (EII) have three functional units : the first unit EIIA receives phosphate from HPr, transfers it to the second unit EIIB, then the third unit EIIC allows the transfer of the phosphoryl group from EIIB to the sugar.
In vivo, the transferred phosphate come from PEP, but in vivo it can come from ATP or acetyl phosphate too.
II. Structure of Enzyme I
| |||||||||||
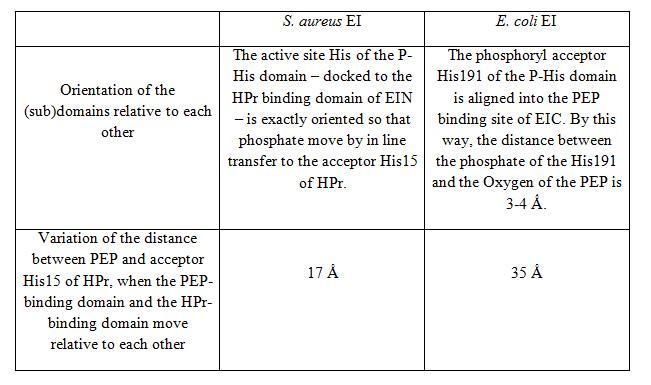
III.Staphylococcus aureus vs Escherichia coli
The X-ray structure of S. aureus EI (SaEI) has been recently solved but structures of other bacterias have been reported before, for instance E. coli EI structure (EcEI). Although SaEI and EcEI X-ray structures are nearly the same, some differences have been observed.
These two X-ray structures could actually be two possible conformations of the Enzyme I, corresponding to two steps of the phosphotransfer from PEP to HPr. EcEI structure correspond to the conformation II and SaEI structure correspond to the conformation I.
Thus, two modest rigid body motions can be described :
Conclusion:
Knowing the structure of S. aureus EI allows a better comprehension of the PTS mechanism. But progresses could be still done such as the possible phenomena which induce the motions of the different domains.
Reference
Oberholzer AE, Schneider P, Siebold C, Baumann U, Erni B. (2009).Crystal structure of enzyme I of the phosphoenolpyruvate:Sugar phosphotransferase system in the dephosphorylated state. J Biol Chem PMID: 19801641
3D Structures of EI
2wqd S. aureus EI phosphorylated structure
2hwg E. coli phosphorylated and Mg2+-oxalate bound structure
Links
Proteopedia page : Enzyme I of the Phosphoenolpyruvate:Sugar Phosphotransferase System (E. coli and S. carnosus)