Maureen E. Hill/Sandbox1
From Proteopedia
Caspase-7 Dynamics
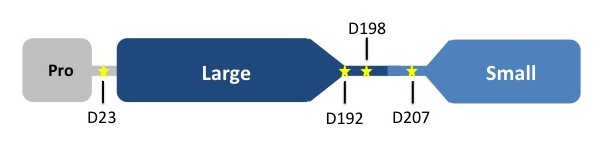
BackgroundCaspases are cysteine-aspartate proteases that are responsible for the execution of apoptosis, also known as programmed cell death. Dysregulation of apoptosis has been linked to neurodegenerative disorders, including Alzheimer's and Huntington's, as well as inflammatory diseases and cancer. The apoptotic caspases consist of two distinct classes: the initiators (caspase -2, -8, -9, and -10) and the executioners (caspase -3, -6, and -7). All caspases are synthesized as catalytically inactive zymogens that must undergo proteolytic cleavage to be activated during apoptosis. Initiator caspases are activated by upstream cellular events, which in turn cleave at distinct internal aspartate residues in the executioner caspases to remove the prodomain and separate the large and small subunits. The executioner caspases then cleave a wide range of targets within the cell that ultimately leads to cellular suicide. Caspase-7 StructureCaspases are crystallized as homodimers. Each monomer contains a large (~20 kDa) and a small (~10 kDa) subunit.
Forms of Caspase-7
at the dimer interface. The allosteric inhibitor binds to C290 within the dimer interface displacing Y223. The displacement of tyrosine from the active site conformation of the enzyme forces R187 into a position that both physically blocks substrate binding, as well as, move the active site cystine 186. Ultimately, these conformational changes inactivate the enzyme.
| |||||||||||